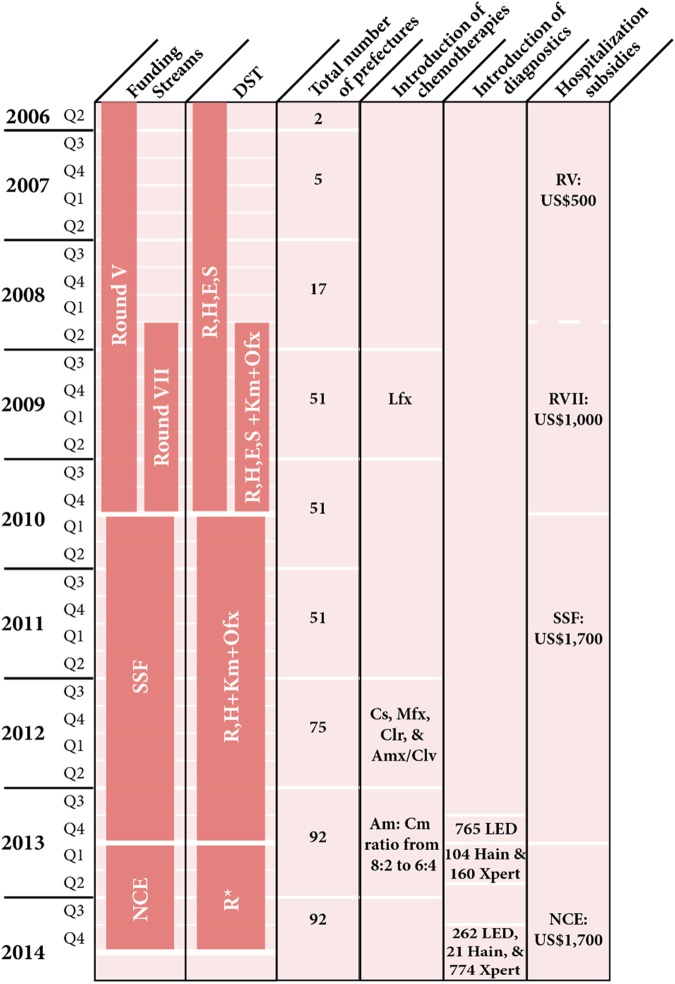
Fig 1. Timeline schematic of China Global Fund MDR-TB programme scale-up.
Quarters (Q) reflect the funding streams and not calendar year. * DST for at least R under NCE only available at selected sites using Xpert, where resistance for R was used as a proxy for MDR-TB. Culture-based DST was run in parallel to adjust treatment options in case of resistance. MDR-TB Regime: Intensive Phase: Z, Km (Am, Cm), Lfx (Mfx), Cs* (PAS, E), Pto; Continuation Phase: Z, Lfx (Mfx), Cs* (PAS, E), Pto. XDR-TB Regime: Intensive Phase: Z, Cm, Mfx, PAS, Cs*, Pto, Clr, Amx/Clv; Continuation Phase: Z, Mfx, PAS, Cs*, Pto, Clr, Amx/Clv. Drug Acronyms: Am = Amikacin, Amx/Clv = Amoxicillin plus clavulanate; Cm = Capreomycin, Clr = Clarithromycin, Cs = Cycloserine, E = Ethambutol, Km = Kanamycin, Lfx = Levofloxacin, Mfx = Moxifloxacin, PAS = p-aminosalicylic acid, Pto = Prothionamide, Z = Pyrazinamide. Drug names in parentheses represent acceptible replacements. Acronyms: DST = Drug-sensitivity testing; Hain = MTBDR(plus) assay machines; LED = LED microscopes; NCE = No cost extension; RV = Round V; RVII = Round VII; SSF = Single-stream funding; Xpert = Xpert MTB/RIF (Cepheid).