Abstract
Evolutionary approaches to culture remain contentious. A source of contention is that cultural mutation may be substantial and, if it drives cultural change, then current evolutionary models are not adequate. But we lack studies quantifying the contribution of mutations to directional cultural change. We estimated the contribution of one type of cultural mutations—modification of memes—to directional cultural change using an amenable study system: learned birdsongs in a species that recently entered an urban habitat. Many songbirds have higher minimum song frequency in cities, to alleviate masking by low-frequency noise. We estimated that the input of meme modifications in an urban songbird population explains about half the extent of the population divergence in song frequency. This contribution of cultural mutations is large, but insufficient to explain the entire population divergence. The remaining divergence is due to selection of memes or creation of new memes. We conclude that the input of cultural mutations can be quantitatively important, unlike in genetic evolution, and that it operates together with other mechanisms of cultural evolution. For this and other traits, in which the input of cultural mutations might be important, quantitative studies of cultural mutation are necessary to calibrate realistic models of cultural evolution.
Keywords: Birdsong, culture, evolutionary models, memes
Cultural change has similarities to genetic evolution, but the study of culture using evolutionary models remains contentious (Aunger 2000; Mesoudi et al. 2006; Henrich et al. 2008). Analogously to genetic evolution, cultural traits are transmitted by replication (i.e., learning), and cultural change involves mutation of cultural traits, drift, and selective processes favoring the persistence of certain cultural traits and the loss of others. Genes and cultural traits also differ in many ways, but many of those differences do not affect evolutionary dynamics in a fundamental way. For example, some cultural traits have a particulate nature similar to genes (termed memes in such cases) and others do not, but this has superficial effects on the outcome of evolutionary modeling (Henrich and Boyd 2002; Mesoudi 2004). Evolutionary models similar to population genetics could, therefore, provide useful tools to study cultural change. An unresolved source of contention, however, concerns the role of cultural mutations in cultural change (Pinker 1997; Henrich et al. 2008). We define cultural mutation as any modification of existing cultural traits or the creation of new cultural traits altogether.
Cultural and genetic mutations differ in several aspects. Importantly, genetic mutations are random with respect to the direction of adaptation and are, thus, on average deleterious and kept at a relatively low incidence in biological systems (Sniegowski and Lensky 1995; Roth et al. 2003). Therefore, genetic mutations do not drive genetic evolution directly, but only provide variants upon which selection and drift then act. Cultural mutations may, on the contrary, result from adaptive cognitive biases and, thus, be common and nonrandom relative to the selective landscape (Laland et al. 2000; Taylor et al. 2009). This has led some to question the utility of modeling cultural change in the same way we model genetic evolution (e.g., Pinker 1997; Bryant 2004), because if a substantial amount of cultural mutations is involved in cultural change, then other evolutionary mechanisms, such as selection and drift, might be less important.
To date we are not able to assess this problem empirically, because we lack studies attempting to quantify the contribution of mutation in cultural change (Reader 2004; Laland and Janik 2006). In some simple systems, such as learned birdsongs, we know that cultural mutations can be important in maintaining memetic diversity (Slater et al. 1980; Lynch and Baker 1994) or keeping cultural variation within the range of species typical traits (Fehér et al. 2009). But we lack studies that evaluate the contribution of cultural mutations in episodes of directional cultural change.
Understanding the role of mutations in cultural evolution is necessary because, if mutations have a more prominent role here than in genetic evolution, then models of cultural evolution need to be constructed to accommodate this, which currently they do not (reviewed in Richerson and Boyd 2005). One reason for the lack of such studies is pragmatic, because units of cultural transmission (i.e., memes) can be difficult to delimit, especially in humans (Aunger 2000; Mesoudi et al. 2006). Most work on culture is made on humans, in which several cultural traits are complex and difficult to quantify and track. Considering simpler systems may, therefore, be pragmatically useful and provides insight into fundamental aspects of cultural evolution. In systems such as oscine birdsong memes are easily identifiable, and thus it is possible to identify their modifications (Lynch et al. 1989).
Oscine songs result from a combination of cultural transmission (Marler 1990) and genetically based physiology and learning rules (Podos et al. 2004; Gardner et al. 2005; Fehér et al. 2009), such that cultural dynamics occur within a range of species-typical phenotypes (Podos et al. 2004). Like many other songbirds, dark-eyed juncos (Junco hyemalis) learn their songs and, in adulthood, sing a small repertoire of stable song types (Marler et al. 1962; Konishi 1964). These song types consist of trilled syllables (Fig. 1) and are transmitted by social learning (Konishi 1964; Newman et al. 2008) forming well-delimited memes. In addition to copying (i.e., learning) memes from conspecifics, juncos may also modify those memes to some extent and can learn new ones that they create during an early phase of socially stimulated improvisation (Marler et al. 1962).
Figure 1.

Examples of dark-eyed junco song types. Spectrograms of two song types, as sung by mountain (left panels) and urban males (right panels). Examples include a song type that differs little in minimum frequency between populations (top panels) and another that differs by a large extent (bottom panels).
Thus, a meme pool of junco songs can change relative to previous generations due to modifications of existing memes (which is a form of cultural mutation) or by replacement of some memes with others. Replacement of memes in a population can happen by a variety of mechanisms: for example, if individuals in one generation learn only a subset of the memes from the previous generation (cultural selection and cultural drift), if they create new memes altogether (another form of cultural mutation), or if the population receives new memes from migrant birds (meme flow, analogous to gene flow). Here we estimate the contribution of one form of cultural mutation—meme modifications—to the directional cultural change of songs that occurred recently in an urban junco population (Slabbekoorn et al. 2007). We focus on the role of meme modifications (i.e., modification of existing song types) because, in our study system, the contribution of this type of cultural mutation is possible to estimate.
Our study system is an urban dark-eyed junco population in south California, founded approximately 30 years ago, which has higher minimum song frequency (pitch) than in the native mountain range (Slabbekoorn et al. 2007). Such increased minimum frequency is common in urban songbird populations (reviewed in Slabbekoorn and Ripmeester 2008), presumably because it alleviates song masking by anthropogenic noise that is progressively louder at lower frequencies (Brumm and Slabbekoorn 2005). Songs in the urban junco population could have changed by modifying the minimum frequency of the existing memes (Bermúdez-Cuamatzin et al. 2009), replacing some of the lower frequency memes by higher frequency ones (Halfwerk and Slabbekoorn 2009) or a combination of both.
We estimated the contribution of meme modifications for this directional change, comparing the minimum frequency of urban songs to the same song types as sung in a population representative of the native mountain range. The data from the mountain range indicate the most likely frequencies of the song types when first entering the urban population, and thus allow estimating by how much memes were modified in the urban population. There can be alternative interpretations such as the urban population having received and selected higher frequency variants of the song types recorded in the mountains. But songbirds are known to modify the frequency of songs in noisy environments (Tumer and Brainard 2007; Bermúdez-Cuamatzin et al. 2009; Luther and Baptista 2010), and thus it is more likely that these song types were modified after, rather than before, entering the urban population. Therefore, we interpret the differences in minimum frequency between the mountain and urban versions of the same song types as meme modifications in the urban population. If meme modifications are as large as the overall population divergence, then the input provided by these meme modifications suffices to explain this divergence. But if meme modifications are smaller in magnitude, then part of the change seen in the urban population must have been due to the replacement of some lower frequency memes by higher frequency ones. This knowledge of the relative contribution of meme modifications versus other forms of cultural change allows us to assess if current models of cultural evolution are realistic and enhances our ability to improve such models.
Methods
We recorded long-range songs of individually marked dark-eyed juncos during the breeding seasons of 2006 and 2007, in an urban population on the campus of the University of California at San Diego and a mountain population in the closest part of the native range (near Mt. Laguna, CA). These populations do not differ in body mass or skeletal size (Rasner et al. 2004), and song frequency (pitch) in juncos is not related to body size (Cardoso et al. 2008). On the contrary, most variation in song frequency is due to differences between song types (Cardoso et al. 2009). The study populations are described in Yeh (2004), and details on capture, marking, and recording procedures are in Cardoso et al. (2007).
In total, we recorded 1017 song bouts from 151 males (101 urban and 50 mountain), comprising 262 different song types (168 urban and 115 mountain, of which 21 were present in both populations). Song types were identified by the shape of the trilled syllable in sound spectrograms (Konishi 1964; Newman et al. 2008) irrespective of absolute frequency (e.g., Fig. 1; many other examples in Newman et al. 2008 and Cardoso et al. 2007, 2008, 2009). On average, we recorded 3.47 different song types per male (urban 3.66 ± 0.19 SE, mountain 3.08 ± 0.23) and 102 song types were sung by multiple males (75 urban and 29 mountain).
We measured minimum frequency of songs as described in Cardoso et al. (2008) and averaged the values for recordings of the same song type sung by the same bird. The minimum frequency of each male is the average minimum frequency of its song types. To compare song types found in the two populations (Fig. 2A) and to test for a relation between song types’ frequencies and the number of males singing them (Fig. 3), we averaged the minimum frequency of song types across the males singing them in each population. For each song type and bird, we also noted the number of elements per syllable, the number of frequency inflections, and the extent of two-voiced sounds, as described in Cardoso et al. (2007). As above, measurements for song types sung by multiple males were averaged across males in each population.
Figure 2.
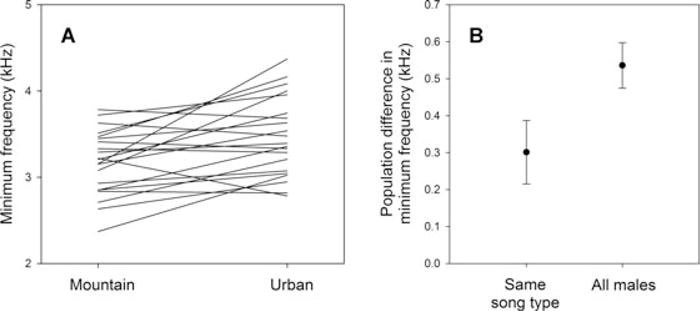
Differences in minimum frequency of the same song types in the mountain and urban populations. (A) Paired differences for each of the 21 song types sung in both populations. (B) Average difference within song type, compared to the overall difference between populations (means ± SE).
Figure 3.
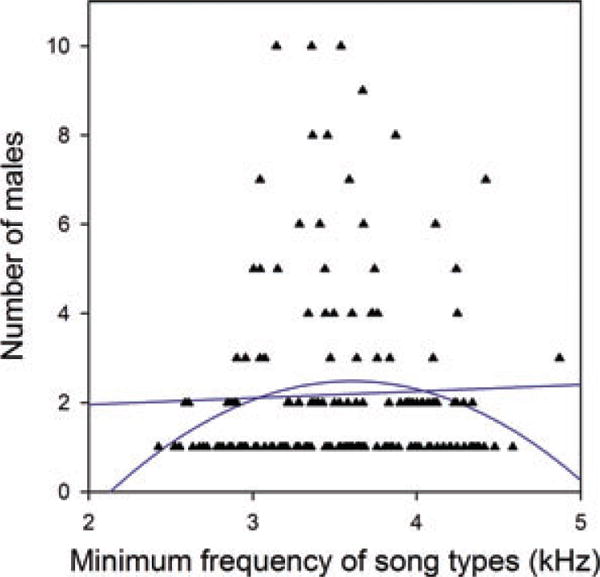
Number of males singing each of the 168 urban song types. The best-fit linear and quadratic lines for these data are shown (see text for details).
To test whether modifications of song types are of a sufficient magnitude to explain the population divergence, we ran a generalized linear mixed model (GLMM) for minimum frequency with males as subjects, and song types as the within-subjects variable. The predictors were population, a dichotomous variable noting if song types were found in both populations or not, and the interaction term. This interaction effect between the two predictors (population and whether song types exist in both populations) is the result of interest and tests whether modifications of song types (i.e., divergence of song types that exist in both populations) are different from the overall magnitude of the population divergence.
Results and Discussion
The average minimum frequency of urban males was 0.536 kHz higher than in mountain males (urban: 3.591 kHz ± 0.038 SE; mountain: 3.056 kHz ± 0.041; independent samples t-test, t = 8.72, N = 151, P < 0.001). Twenty-one song types were sung in both populations (Fig. 1), and we compared their minimum frequency to estimate by how much memes were modified in the urban population. The minimum frequency of these song types was higher in the urban than in the mountain population (Fig. 2A; paired samples t-test, t = 3.51, N = 21, P = 0.007) and their paired difference was 0.301 kHz ± 0.086. This is significantly smaller than the overall difference between populations (GLMM interaction term, Wald χ2 = 6.09, P = 0.014), and corresponds to 56% of the magnitude for the overall difference between the populations (Fig. 2B).
These findings indicate that, on average, dark-eyed juncos modified the minimum frequency of song types substantially, but that the average extent of these modifications is still much lower than the population divergence in minimum frequency. The best estimate is that variation provided by this type of meme mutations can explain 56% of the magnitude of the population divergence (Fig. 2B) and that the remaining is due to the replacement of lower frequency memes by higher frequency ones. Thus, unlike in genetic evolution, the input of repeated cultural mutations can be quantitatively important, but still operate together with other mechanisms of cultural evolution.
Increasing the minimum frequency of memes could also have modified them to an extent that song types are no longer recognized as the same, which, if true, could cause us to underestimate meme modifications. Junco song types are identified by the shape of the trilled syllable in sound spectrograms (Konishi 1964; Newman et al. 2008), and conceivably the minimum frequency could be increased by simplifying the shape of syllables (e.g., omitting the lower frequency elements or the lower voice in two-voiced sounds) or modifying the frequency inflections along the syllable, in which case the song types would not be recognized as the same any more. But we found no evidence for these changes in the urban population: number of elements and frequency inflections did not differ between populations (elements per syllable: urban 1.49 ± 0.40, N = 168 song types, mountain 1.49 ± 0.48, N = 115, t = 0.01, P = 0.99; frequency inflections per syllable: urban 0.89 ± 0.045, mountain 0.95 ± 0.053, t = 0.91, P = 0.36) and the extent of two-voiced sounds was larger, not smaller, in the urban population (urban: 8.24 msec ± 0.72, mountain: 6.07 msec ± 0.55, t = 2.22, P = 0.027).
Currently, there seems to be no cultural selection for higher frequency memes in the urban population. If song types with higher minimum frequency were currently being culturally selected (i.e., being preferentially learned), we would expect that, on average, those song types were sung by more males. Many song types in the urban population were sung by a single male (55% of song types, a value consistent with previous studies, Newman et al. 2008), but others were sung by multiple males (Fig. 3). However, the minimum frequency of the song types was not correlated with the number of males singing them (Fig. 3; r = 0.038, N = 168, P = 0.629). On the contrary, the best-fit quadratic curve relating the minimum frequency of song types with the number of males singing them (Fig. 3; F2,165 = 2.553, P = 0.081) has its maximum at 3.605 kHz, which is identical to the population average (3.591 kHz, see above).
Thus, after an increase of circa 0.5 kHz relative to the native range, the average minimum frequency of the urban population appears to be presently stable. This large increase in minimum frequency (one of the largest reported for urban birdsongs, cf. Slabbekoorn and Boer-Visser 2006; Hu and Cardoso 2010) is best explained by the ability of dark-eyed juncos to both modify memes (mutation) and choose from a variety of alternative memes. This has similarities to the mechanism of adaptive genetic evolution (mutation plus selection), but with three important differences.
First, although the quantitative contribution of genetic mutations driving evolution is negligible (Sniegowski and Lensky 1995), we found that modifications of memes were common and estimated that their input explains about half the magnitude of the population divergence. Second, these meme mutations appear to have been directional (Fig. 2A), although this apparent directionality could also have been the result of selection eliminating mutations in the opposite direction. Even though we did not show that mutations are directional in this system, it is likely that they are, because birds singing in noisy conditions are capable of frequency adjustments postlearning (Tumer and Brainard 2007) and this is thought to play a role in the adaptation to urban noise (Patricelli and Blickley 2006; Bermúdez-Cuamatzin et al. 2009). Third, in this species, memes can be replaced not only by cultural selection or drift, but also by de novo creation of memes (learning new song types from improvisation, Marler et al. 1962), which is another form of cultural mutation. Because many urban song types were sung by a single male, new song types appear to contribute to maintain a diverse meme pool and, thus, should have influenced the pattern of meme replacement.
These three findings indicate that cultural mutations can be quantitatively important and influence the direction of cultural change. It has been argued that such a role of cultural mutations is an impediment to study culture in an evolutionary framework (e.g., Pinker 1997; Bryant 2004), because then evolutionary mechanisms that we typically think of as more powerful, such as selection or drift, might not be important in accounting for cultural change. The counter-argument was also made that evolutionary models could accommodate high rates of cultural mutation, even directional mutation (e.g., Mesoudi et al. 2006). Our results support the above concern to some extent, as they indicate that the contribution of mutations in cultural change can be much more important than in genetic evolution. But, in line with the counter-argument, we also show that the input of cultural mutations can operate together with other mechanisms of cultural evolution and that its contribution is subject to quantitative study. As such, when necessary, the contribution of cultural mutations could be incorporated into improved models of cultural evolution.
Many researchers are quantifying aspects of cultural dynamics necessary to build realistic evolutionary models (e.g., learning and cultural diffusion, Smith et al. 2008). Some models of cultural evolution for birdsong also recognize frequent cultural mutations as necessary to maintain song diversity (Lynch et al. 1989; Lynch and Baker 1994; Lachland and Slater 1999), but, in a more-general context, studies of the role of mutations in cultural change are surprisingly lacking (Reader 2004; Laland and Janik 2006). We suggest that quantifying the origin and magnitude of cultural variants should be an early step in the study of cultural evolution and, when mutation is found to be common, this should be used to build more realistic evolutionary models.
Acknowledgments
We thank T. Price and E. Ketterson for many helpful discussions on this manuscript, Y. Hu for assistance in song measurements, and R. Lande, H. Hoekstra and K. Marchetti for logistical support at the University of California at San Diego. This study was supported by the Fundação para a Ciência e a Tecnologia grant SFRH/BPD/21509/2005 to GCC, and a National Science Foundation graduate research fellowship and DDIG grant (DEB-0808284) to JWA.
LITERATURE CITED
- Aunger R, editor. Darwinizing culture. Oxford Univ. Press; Oxford, U.K: 2000. [Google Scholar]
- Bermúdez-Cuamatzin E, Ríos-Chelén AA, Gil D, Garcia CM. Strategies of song adaptation to urban noise in the house finch: syllable pitch plasticity or differential syllable use? Behaviour. 2009;146:1269–1286. [Google Scholar]
- Brumm H, Slabbekoorn H. Acoustic communication in noise. Adv Study Behav. 2005;35:151–209. [Google Scholar]
- Bryant JM. An evolutionary social science? A skeptic’s brief, theoretical and substantive. Philos Soc Sci. 2004;34:451–492. [Google Scholar]
- Cardoso GC, Atwell JW, Ketterson ED, Price TD. Inferring performance in the songs of Dark-eyed juncos (Junco hyemalis) Behav Ecol. 2007;18:1051–1057. [Google Scholar]
- Cardoso GC, Mamede AT, Atwell JW, Mota PG, Ketterson ED, Price TD. Song frequency does not reflect differences in body size among males in two oscine species. Ethology. 2008;114:1084–1093. [Google Scholar]
- Cardoso GC, Atwell JW, Ketterson ED, Price TD. Song types, song performance, and the use of repertoires in dark-eyed juncos (Junco hyemalis) Behav Ecol. 2009;20:901–907. [Google Scholar]
- Fehér O, Wang H, Saar S, Mitra PM, Tchernichovski O. De novo establishment of wild-type song culture in the zebra finch. Nature. 2009;459:564–568. doi: 10.1038/nature07994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner TJ, Naef F, Nottebohm F. Freedom and rules: the acquisition and reprogramming of a bird’s learned song. Science. 2005;308:1046– 1049. doi: 10.1126/science.1108214. [DOI] [PubMed] [Google Scholar]
- Halfwerk W, Slabbekoorn H. A behavioural mechanism explaining noise-dependent frequency use in urban birdsong. Anim Behav. 2009;78:1301–1307. [Google Scholar]
- Henrich J, Boyd R. On modeling cognition and culture: why cultural evolution does not require replication of representations. J Cogn Cult. 2002;2:87–112. [Google Scholar]
- Henrich J, Boyd R, Richerson PJ. Five misunderstandings about cultural evolution. Hum Nat. 2008;19:119–137. doi: 10.1007/s12110-008-9037-1. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cardoso GC. Which birds adjust the frequency of vocalizations in urban noise? Anim Behav. 2010;79:863–867. [Google Scholar]
- Konishi M. Song variation in a population of Oregon juncos. Condor. 1964;66:423–436. [Google Scholar]
- Lachland RF, Slater PJB. The maintenance of vocal learning by gene-culture interaction: the cultural trap hypothesis. Proc R Soc Lond B. 1999;266:701–706. [Google Scholar]
- Laland KN, V, Janik M. The animal cultures debate. Trends Ecol Evol. 2006;21:542–547. doi: 10.1016/j.tree.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Laland KN, Odling-Smee J, Feldman MW. Niche construction, biological evolution, and cultural change. Behav Brain Sci. 2000;23:131–175. doi: 10.1017/s0140525x00002417. [DOI] [PubMed] [Google Scholar]
- Luther D, Baptista L. Urban noise and the cultural evolution of bird songs. Proc R Soc Lond B. 2010;277:469–473. doi: 10.1098/rspb.2009.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A, Plunkett GM, Baker AJ, Jenkins PF. A model of cultural evolution of chaffinch song derived with the meme concept. Am Nat. 1989;133:634–653. [Google Scholar]
- Lynch A, Baker AJ. A population memetics approach to cultural-evolution in chaffinch song-differentiation among populations. Evolution. 1994;48:351–359. doi: 10.1111/j.1558-5646.1994.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Marler P. Song learning: the interface between behaviour and neuroethology. Phil Trans R Soc B. 1990;329:109–114. doi: 10.1098/rstb.1990.0155. [DOI] [PubMed] [Google Scholar]
- Marler P, Kreith M, Tamura M. Song development in hand-raised Oregon juncos. Auk. 1962;79:12–30. [Google Scholar]
- Mesoudi A. Is human cultural evolution Darwinian? Evidence reviewed from the perspective of The Origin of Species. Evolution. 2004;58:1–11. doi: 10.1111/j.0014-3820.2004.tb01568.x. [DOI] [PubMed] [Google Scholar]
- Mesoudi A, Whiten A, Laland KN. Towards a unified science of cultural evolution. Behav Brain Sci. 2006;29:329–383. doi: 10.1017/S0140525X06009083. [DOI] [PubMed] [Google Scholar]
- Newman MM, Yeh PJ, Price TD. Song variation in a recently founded population of the dark-eyed junco (Junco hyemalis) Ethology. 2008;114:164–173. [Google Scholar]
- Patricelli GL, Blickley JS. Avian communication in urban noise: causes and consequences of vocal adjustment. Auk. 2006;123:639–649. [Google Scholar]
- Pinker S. How the mind works. W. W. Norton; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Podos J, Huber SK, Taft B. Bird song: the interface of evolution and mechanism. Annu Rev Ecol Evol Syst. 2004;35:55–87. [Google Scholar]
- Rasner CA, Yeh P, Eggert LS, Hunt KE, Woodruff DS, Price TD. Genetic and morphological evolution following a founder event in the dark-eyed junco, Junco hyemalis thurberi. Mol Ecol. 2004;13:671–681. doi: 10.1046/j.1365-294x.2004.02104.x. [DOI] [PubMed] [Google Scholar]
- Reader SM. Distinguishing social and asocial learning using diffusion dynamics. Learn Behav. 2004;32:90–104. doi: 10.3758/bf03196010. [DOI] [PubMed] [Google Scholar]
- Richerson PJ, Boyd R. Not by genes alone: How culture transformed human evolution. University of Chicago Press; Chicago: 2005. [Google Scholar]
- Roth JR, Kofoid E, Roth FP, Berg OG, Seger J, Andersson DI. Regulating general mutation rates: examination of the hypermutable state model for Cairnsian adaptive mutation. Genetics. 2003;163:1483–1496. doi: 10.1093/genetics/163.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slabbekoorn H, Boer-Visser A. Cities change the songs of birds. Current Biol. 2006;16:2326–2331. doi: 10.1016/j.cub.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Ripmeester EAP. Birdsong and anthropogenic noise: implications and applications for conservation. Mol Ecol. 2008;17:72–83. doi: 10.1111/j.1365-294X.2007.03487.x. [DOI] [PubMed] [Google Scholar]
- Slabbekoorn H, Yeh P, Hunt K. Sound transmission and song divergence: a comparison of urban and forest acoustics. Condor. 2007;109:67–78. [Google Scholar]
- Slater PJB, Ince SA, Colgan PW. Chaffinch song types: their frequencies in the population and distribution between the repertoires of different individuals. Behaviour. 1980;75:207–218. [Google Scholar]
- Smith K, Kalish ML, Griffiths TL, Lewandowsky S. Introduction: Cultural transmission and the evolution of human behaviour. Phil Trans R Soc Lond B. 2008;363:3469–3476. doi: 10.1098/rstb.2008.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniegowski PD, Lensky RE. Mutation and adaptation: the directed mutation controversy in evolutionary perspective. Annu Rev Ecol Syst. 1995;26:553–578. [Google Scholar]
- Taylor AH, Hunt GR, Medina FS, Gray RD. Do New Caledonian crows solve physical problems through causal reasoning? Proc R Soc Lond B. 2009;276:247–254. doi: 10.1098/rspb.2008.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumer EC, Brainard MS. Performance variability enables adaptive plasticity of ‘crystallised’ adult birdsong. Nature. 2007;450:1240–1245. doi: 10.1038/nature06390. [DOI] [PubMed] [Google Scholar]
- Yeh PJ. Rapid evolution of a sexually selected trait following population establishment in a novel habitat. Evolution. 2004;58:166–174. doi: 10.1111/j.0014-3820.2004.tb01583.x. [DOI] [PubMed] [Google Scholar]


