Abstract
Purpose
In contrast to most other mammals, canine oocytes are ovulated in an immature state and undergo oocyte maturation within the oviduct during the estrus stage. The aim of the study was to investigate whether oviduct cells from the estrus stage affect the maturation of oocytes and show gene expression patterns related to oocyte maturation.
Methods
We analyzed MAPK1/3, SMAD2/3, and BMP6/15 expression in oviduct cells, cumulus cells, and oocytes from anestrus, estrus, and diestrus stages. Next, we investigated the effect of co-culture with oviduct cells derived from the estrus stage upon in vitro maturation (IVM) of canine oocytes.
Results
There was significantly higher MAPK1/3 (1.42 ± 0.02 and 2.23 ± 0.06), SMAD2/3 (0.77 ± 0.03 and 2.39 ± 0.07), and BMP15 (2.21 ± 0.16) expression in oviduct cells at the estrus stage (P < 0.05). In cumulus cells, MAPK1 (1.26 ± 0.07), SMAD2/3 (0.82 ± 0.01, 1.04 ± 0.01), and BMP6 (13.09 ± 0.11) expression was significantly higher in the estrus stage (P < 0.05). In oocytes, significant upregulation of MAPK1/3 (14,960 ± 3121 and 1668 ± 253.4), SMAD3 (774.6 ± 79.62), and BMP6 (8500 ± 895.4) expression was found in the estrus stage (P < 0.05). After 72 h of IVM culture, a significantly higher maturation rate was observed in oocytes co-cultured with oviduct cells (10.0 ± 1.5%) than in the control group (3.2 ± 1.4%).
Conclusions
We demonstrate that oviduct cells at the estrus stage highly expressed MAPK1/3, SMAD2/3, and BMP15. Furthermore, canine oviduct cells from the estrus stage enhance the culture environment for canine oocyte maturation.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0910-x) contains supplementary material, which is available to authorized users.
Keywords: Co-culture, Estrus stage, Gene expression, In vitro maturation, Oocyte, Oviduct cell
Introduction
The application of assisted reproductive technology has been beneficial in many mammalian species for which techniques such as in vitro maturation (IVM) of oocytes or in vitro culture (IVC) of embryos are available [1]. In dogs, however, IVM and IVC are not well established because of the unique reproductive characteristics of this species. In most mammals, oocyte maturation occurs within the ovarian follicle, and then the mature oocytes are ovulated and fertilized within the oviduct. However, canine oocytes are ovulated at an immature state that requires a further 48 to 72 h for completion of meiosis within the oviduct [2, 3] before they undergo fertilization and begin early embryo development. Because of this unique reproductive physiology characteristic and the paucity of information concerning factors regulating post-ovulatory oocyte maturation, the efficiency of canine IVM is low.
In order to improve canine IVM efficiency, various experiments have been performed. For example, oviduct cells were harvested from bitches regardless of their estrous cycle stages for co-culture in a modified tissue culture medium supplemented with bovine serum albumin (BSA), but a rate of only 9% metaphase I (MI)/anaphase I (AI)/metaphase II (MII) was observed [4]. Also, various kinds of gonadotropins such as follicle-stimulating hormone (FSH) and luteinizing hormone (LH) or estradiol-17β and epidermal growth factors (EGF) were added, which significantly increased the MII rate, up to 7.2% compared with controls [5–7]. In addition, the canine fibroblasts [8] or granulosa cells [9] were used for co-culture and the MII rate was again significantly increased, up to 11%. Despite these efforts, the efficacy of IVM is still much lower compared with other animals, such as mice (68.3%), pig (87.8%), and cattle (79.2%) [10–12].
This low efficiency may be due to an insufficient understanding of the cellular signaling pathways or gene expression that occurs during canine oocyte maturation. The roles of mitogen-activated protein kinases (MAPKs) in oogenesis, particularly those involved in meiosis progression, have been examined in several studies. For example, MAPK is required for resumption of meiosis in rat cumulus-oocyte complexes (COCs) [13]. Furthermore, by knocking out MAPK1/3 activities in mice, poorly assembled MII spindles were observed [14], which indicates that MAPK1/3 activities are required for meiotic maturation. In addition, a study showed that inhibiting SMAD2/3 signaling disrupted oocyte-to-cumulus communication and severely reduced the inner cell mass in blastocysts derived from oocytes matured in the presence of SMAD2/3 inhibitors, which indicates that communication between the oocyte and cumulus via SMAD2/3 is essential to allow exchange of factors needed for oocyte development [15]. Also, bone morphogenetic proteins 6/15 (BMP6/15) are oocyte-secreted factors that play a crucial role in regulating ovarian follicular development. It has been proved that when COCs were treated with increasing doses of BMP6/15, their apoptotic level was significantly decreased [16]. Likewise, a number of studies have revealed that activation of MAPK1/3, SMAD2/3, and BMP6/15 plays a key role in oocyte maturation by actions on granulosa and cumulus cells in other mammals [17, 18]. However, no systematic research for these genes has been performed to identify the role of granulosa and cumulus cells and oviduct cells in dog.
Accordingly, we analyzed the oocyte maturation-related genes in canine cumulus cells, oocytes, and oviduct cells. Because the canine oocytes have completed the maturation process within the oviduct, we hypothesized that genes related to oocyte maturation might be expressed in canine oviduct cells from the estrus stage. The aim of the present study was to evaluate (1) the expression patterns of MAPK1, MAPK3, SMAD2, SMAD3, BMP6, and BMP15 in cumulus cells, oocytes, and oviduct cells derived from anestrus, estrus, and diestrus stages of the estrus cycles in dogs and (2) the effects of co-culture of canine COCs with the cells in which these genes are highly expressed during IVM on canine nuclear maturation.
Material and methods
Animal use in experiments
In this study, 1- to 3-year-old mixed breed bitches were used for collection of oviduct cells, cumulus cells, and oocytes. In order to collect these cells, we used four bitches in the anestrus stage, eight bitches in the estrus stage, and three bitches in the diestrus stage. Additionally, in order to collect cumulus cells surrounding in vivo matured oocytes (mature cumulus cells, MCCs), nine dogs in the estrus stage were used. In order to study IVM, three bitches in the estrus stage were used to collect immature oocytes. All experiments and studies were conducted in accordance with recommendations described in “The Guide for the Care and Use of Laboratory Animals” published by the Institutional Animal Care and Use Committee of Seoul National University (approval number: SNU-140704-1). In this respect, dog care facilities and the procedures performed met or exceeded the standards established by the Committee for Accreditation of Laboratory Animal Care at Seoul National University.
Determination of estrous cycle stage
The estrous cycle was divided into anestrus, estrus, and diestrus stages. To determine the specific stage of the cycle, we evaluated daily basal progesterone (P4) concentrations and vaginal cytology. In order to measure these parameters, samples were collected every day from the bitches for 3 to 7 days. Blood was collected by cephalic venipuncture and allowed it to clot. After centrifuging it at 1960g for 10 min, the serum was aspirated. The P4 level was immediately measured with a DSL-3900 Active P4 Coated-Tube Radioimmunoassay Kit (Diagnostic Systems Laboratories, Inc., Webster, TX, USA) and classified as anestrus (<1 ng/mL), estrus (4 to 10 ng/mL), or diestrus (15 to 80 ng/mL) stage [19, 20]. The vaginal smears were performed using sterilized swabs. Smears were obtained by inserting a sterile swab into the lips of the vulva, and then the swab was rolled on a glass slide. After staining the slide using a Diff-Quik® kit (International Reagents Corp., Kobe, Japan), it was examined (Supplementary material 1) [21].
Collection of immature oocytes and cumulus cells
Canine oocytes and cumulus cells were obtained by ovariohysterectomy (OHE) as previously described [22]. Briefly, anesthesia was induced with 6 mg/kg ketamine HCl and 1 mg/kg xylazine, and general anesthesia was maintained with 2% isoflurane. While in dorsal recumbency, bitches were aseptically prepared for surgery, and a midline ventral incision was made to expose the reproductive tract for ovary collection. The duration of surgery was kept to between 30 and 40 min, and the actual time taken was recorded. Canine ovaries were transported to the laboratory within 1 h at 37 °C in saline. COCs were obtained as previously described [23]. In brief, ovaries were washed several times with phosphate-buffered saline (PBS; Invitrogen, Carlsbad, CA, USA) and minced with a surgical blade in HEPES-buffered tissue culture medium 199 (TCM-199; Invitrogen, Carlsbad, CA, USA) supplemented with 2 mmol/L NaHCO3, 5 mg/mL bovine serum albumin, and 1% penicillin-streptomycin. Then, COCs with more than two layers of cumulus cells and with homogeneous cytoplasm >100 μM in diameter were selected [23, 24]. In order to separate cumulus cells and immature oocytes, the COCs were denuded by vortexing in 0.1% (w/v) hyaluronidase. After vortexing, the denuded oocytes were stored at −80 °C before use for RNA extraction and cumulus cells were cultured up to passage 2 in RCMEP (Stem Cell Research Center, Biostar, Seoul, Republic of Korea) containing 0.2 mmol/L ascorbic acid, 0.09 mmol/L calcium, 5 ng/mL EGF, and 5% fetal bovine serum (FBS) [25]. When the samples had reached about 100% confluency, they were harvested and centrifuged at 1500 rpm for 2 min. After removing the supernatant, cell pellets were stored at −80 °C until used for RNA extraction.
Collection and culture of oviduct cells
Oviduct cells were retrieved from anesthetized females following the slight oviduct flushing method [26]. Briefly, through an ovarian bursal slit, a cannula consisting of an inverted flanged bulb needle, mounted on an empty sterilized 10 mL syringe without the plunger, was inserted into the oviduct to its end. Then, a fine hypodermic needle (24 gauge) filled with 10 mL of sterilized normal saline was cannulated into just above the uterotubal junction at the base of the oviductal canal, and finally, oviduct cells were collected by retrograde flushing with appropriate pressure. The flushing medium containing oviduct cells was transported to the laboratory within 30 min. Then, the flushing medium was centrifuged at 1960g for 2 min and the supernatant was removed. Thereafter, 3 mL of RCMEP medium was added to the tube and centrifuged at 1960g for 2 min, and then cells were resuspended in the RCMEP medium. The oviduct cells were cultured following the same protocol for cumulus cells as described above.
Total RNA extraction and complementary DNA synthesis
Total RNA was extracted from 2 × 106 cells/mL oviduct cells, 2 × 106 cells/mL cumulus cells, and 300 oocytes derived from each of the three estrous cycle stages (anestrus, estrus, and diestrus) using the Easy-spin™ (DNA-free) Total RNA Extraction Kit (iNtRON Biotechnology, Inc., Kyunggi, Republic of Korea) following the manufacturer’s instructions. Total RNA from all samples was eluted according to the manufacturer’s protocol (iNtRON Biotechnology, Inc.). Total RNA concentration was measured using spectrophotometry (NanoDrop 2000, Thermo Fisher Scientific, Inc., Waltham, MA, USA), and samples were immediately stored at −80 °C until complementary DNA (cDNA) synthesis. Total RNA was reverse transcribed into cDNA using amfiRivert II cDNA Synthesis Premix (GenDEPOT, Barker, TX, USA) according to the manufacturer’s instructions.
Real-time PCR
The primers for MAPK1, MAPK3, SMAD2, SMAD3, BMP6, BMP15, and β-actin genes were designed from sequences of canine genes obtained from NCBI; all primer sequences were standardized using a standard curve and are listed in Table 1. Real-time PCR was performed using an ABI 7300 Real-Time PCR System (Applied Biosystems, Forest City, CA, USA) according to the manufacturer’s instructions with minor modification. The total volume PCR reaction mixture was 20 μL in a real-time PCR plate (MicroAmp optical 96-well reaction plate, Singapore), and the mixture was composed of 2 μL cDNA, 0.4 μL forward primer, 0.4 μL reverse primer, 10 μL SYBR Green interaction dye (Takara Bio USA, Inc., Mountain View, CA, USA), and 7.2 μL diethyl pyrocarbonate water. For each sample, a typing result was considered valid where (1) an amplification curve with a crossing point of less than 35 cycles was observed upon quantification analysis and (2) melting peaks of 75–85 °C were observed during analysis. The thermal program consisted of an initial denaturation step of 94 °C for 10 min followed by 40 cycles of 95 °C for 15 s, annealing for 1 min at 60 °C, and extension for 1 min at 72 °C. For relative analysis of gene expression, MCCs were used as a reference sample. The mature COCs were collected as described in previous reports [27, 28], and the MCCs were separated from COCs by repeated pipetting in 0.1% (v/v) hyaluronidase. All samples were prepared in three replications using real-time PCR.
Table 1.
Sequence-specific primers used for quantification of differentially expressed transcripts
| Gene | Primer sequences (5′→3′) | GenBank No. | Product size (bp) |
|---|---|---|---|
| Beta-actin | F-CCCAGCACAATGAAGATCAA R-ACTCCTGCTTGCTGATCCAC |
XM_005621019.1 | 121 |
| MAPK1 | F-TGTGCCCTAGAACTGCTCCT R-TCCGATCTCTGAGGCTGAGT |
NM_001110800.1 | 144 |
| MAPK3 | F-ACAGTCTCTGCCCTCCAAGA R-GATGAGCCAGTGCTTCTTCC |
XM_005621357.1 | 140 |
| SMAD2 | F-GGAACAACCCTGCTTGTGTT R-GGCCGGATAAAGGGATACAT |
XM_005622832 | 140 |
| SMAD3 | F-GCAAGTGTAGGCGACAGTCA R-TAAGATGAAGGGGTCCATCG |
XM_005638182.1 | 126 |
| BMP6 | F-CTAAGCTGAACGCCATCTCC R-GCATCCACAAGCTCTGACAA |
XM_005640158.1 | 95 |
| BMP15 | F-GACCCTTGGCGAATATAGCA R-CGGTAAACCACAATGGCTCT |
XM_003640274.2 | 118 |
In vitro maturation
For IVM, ovaries were obtained from estrus stage bitches by OHE and immature COCs were collected from the ovaries following the same protocol as described above. The COCs were cultured in an IVM medium comprising tissue culture medium 199 supplemented with 10 ng/mL EGF, 100 nM cysteamine, 0.9 mM sodium pyruvate, 10% FBS, 5 ng/mL FSH, and 1 ng/mL 17-β-estradiol in 12-well plates at 39 °C in a humidified atmosphere of 5% CO2. In order to perform the co-culture experiment, canine oviduct cells were used when they had reached about 70% confluence in 12-well plates (Falcon, Becton Dickinson Ltd., Plymouth, UK) with the RCMEP medium. The medium was replaced with the IVM medium when co-culture was performed. The 12-well plates were supported with 1.0 μm Transwell polyester membrane inserts (Corning, Inc., Pittston, PA, USA) to produce an environment similar to that in in vivo state as closely as possible [29] for 72 h at 39 °C in a humidified atmosphere of 5% CO2 in the IVM medium. The intercellular distance for communication was approximately 2 mm [29]. The COCs were randomly allocated among canine oviduct cell co-culture groups in Transwell polyester membrane inserts or cultured in the IVM medium only (control group). The control group was maintained under the same conditions as the co-culture groups except it was cultured without supporting canine oviduct cells.
Evaluation of oocyte maturation
At the end of IVM, the COCs were denuded with 0.1% hyaluronidase by gentle pipetting and the denuded oocytes were fixed in 4% formaldehyde solution. For assessing in vitro maturation, oocytes were stained with 5 μg/mL Hoechst 33342 for 10 min. The stained oocytes were mounted on a glass slide with a 100% of glycerol drops. The chromatin state and position of nucleus were assessed under a stereomicroscope with UV light to determine the meiotic stages as previously described [30], and oocytes with an extruded first polar body representing the MII stage were identified as shown in Fig. 4. Also, the comparison of messenger RNA (mRNA) expression in oviduct cells, cumulus cells, and oocytes between the co-culture and the control groups would be helpful to evaluate the oocyte maturation.
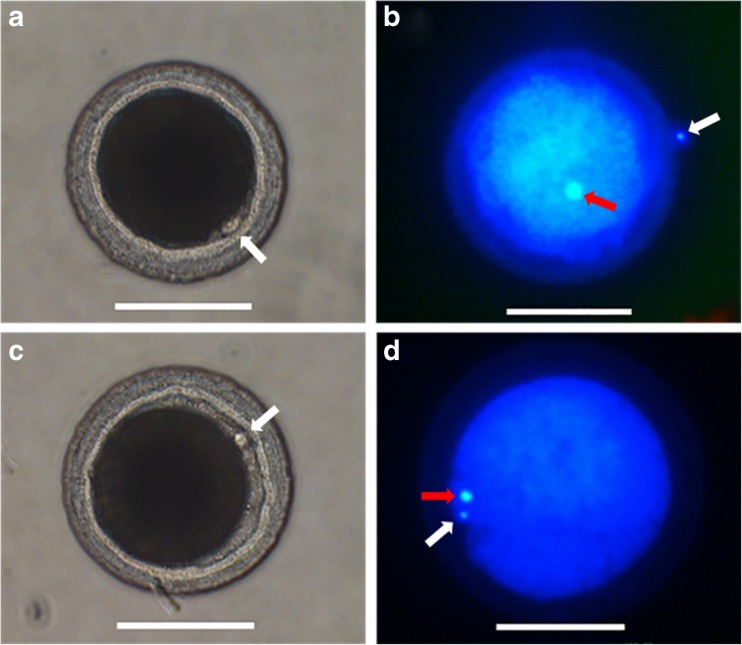
Fig. 4.
First polar body extrusion and chromatin configuration in canine oocytes. a First polar body extrusion in the control group. b Metaphase II (MII) in the control group. c First polar body extrusion in the oviduct cell (OCs) group. d MII in the OCs group. Red arrows MII plate; white arrows first polar body. Scale bar = 100 μm
Statistical analysis
Data for comparative gene expression were analyzed by one-way ANOVA followed by Tukey’s multiple compar-ison test, and an unpaired t test was used for statistical analysis of the in vitro maturation rate. All data were analyzed with GraphPad Prism 5.0 (GraphPad, San Diego, CA, USA), and differences of P < 0.05 were considered significant.
Results
Gene expression of MAPK1, MAPK3, SMAD2, SMAD3, BMP6, and BMP15 in oviduct cells during anestrus, estrus, and diestrus stages
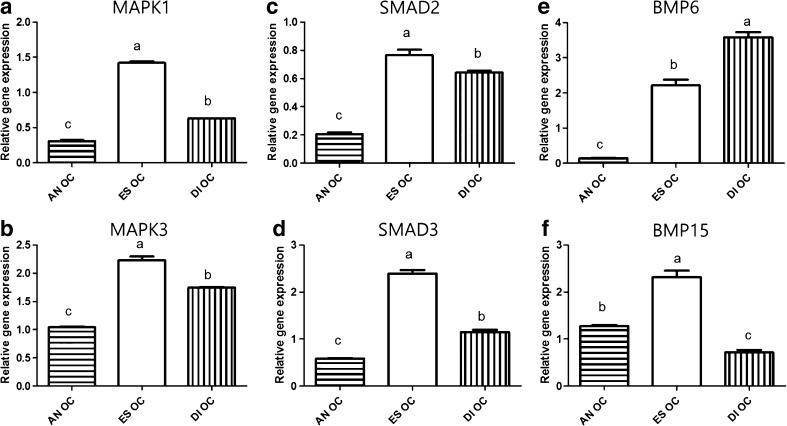
MAPK1 and MAPK3 in oviduct cells (OCs) showed the highest expression in the estrus stage (1.42 ± 0.02 and 2.23 ± 0.06, P < 0.05, Fig. 1a, b) compared with the other two stages. There was significant upregulation of SMAD2 and SMAD3 expression in OCs at the estrus stage (0.77 ± 0.03 and 2.39 ± 0.07, P < 0.05, Fig. 1c, d). While expression of BMP6 in OCs was significantly increased at the diestrus stage (Fig. 1e), BMP15 gene expression was highest in OCs at the estrus stage (2.21 ± 0.16, P < 0.05, Fig. 1f).
Fig. 1.
The relative mRNA expression of MAPK1, MAPK3, SMAD2, SMAD3 BMP6, and BMP15 standardized with mRNA expression of beta-actin in oviduct cells (OCs) during the anestrus, estrus, and diestrus stages. Gene expression of MAPK1 and MAPK3 in OCs during the estrus cycle (a, b). Gene expression of SMAD2 andSMAD3 in OCs during the estrus cycle (c, d). Gene expression of BMP6 and BMP15 in OCs during the estrus cycle (e, f). Values are means ± standard error of the mean from three independent samples of oviduct cells. Different superscripts (a–c) represent significant differences among groups (P < 0.05). AN OC oviduct cells in anestrus stage, ES OC oviduct cells in estrus stage, DI OC oviduct cells in diestrus stage
Gene expression of MAPK1, MAPK3, SMAD2, SMAD3, BMP6, and BMP15 in cumulus cells during anestrus, estrus, and diestrus stages
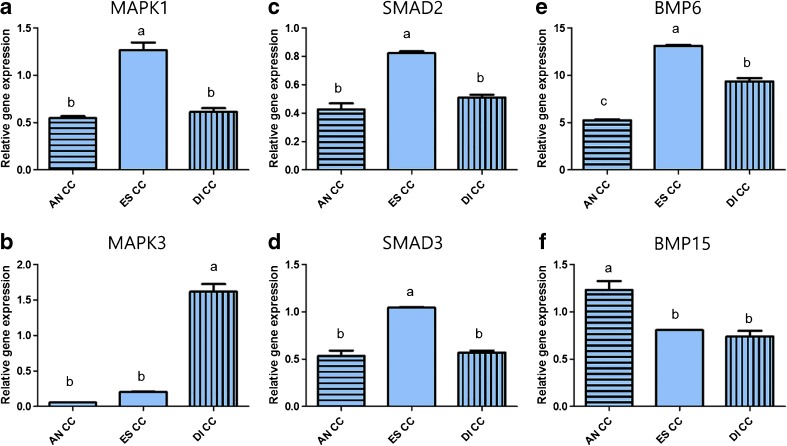
Cumulus cells (CCs) showed the highest MAPK1 expression at the estrus stage (1.26 ± 0.07, P < 0.05, Fig. 2a), while MAPK3 expression was highest in the diestrus stage (1.61 ± 0.10, P < 0.05, Fig. 2b). SMAD2 and SMAD3 expression in CCs was significantly higher at the estrus stage (0.82 ± 0.01, 1.04 ± 0.01, P < 0.05, Fig. 2c, d). BMP6 expression in CCs was significantly upregulated in the estrus stage (13.09 ± 0.11, P < 0.05, Fig. 2e), while BMP15 expression showed the highest level at the anestrus stage (Fig. 2f).
Fig. 2.
The relative mRNA expression of MAPK1, MAPK3, SMAD2, SMAD3, BMP6, and BMP15 standardized with mRNA expression of beta-actin in cumulus cells (CCs) during the anestrus, estrus, and diestrus stages. Gene expression of MAPK1 and MAPK3 in CCs during the estrus cycle (a, b). Gene expression of SMAD2 and SMAD3 in CCs during the estrus cycle (c, d). Gene expression of BMP6 and BMP15 in CCs during the estrus cycle (e, f). Values are means ± standard error of the mean from three independent samples of cumulus cells. Different superscripts (a–c) represent significant differences among groups (P < 0.05). AN CC cumulus cells in anestrus stage, ES CC cumulus cells in estrus stage, DI CC cumulus cells in diestrus stage
Gene expression of MAPK1, MAPK3, SMAD2, SMAD3, BMP6, and BMP15 in immature oocytes during anestrus, estrus, and diestrus stages
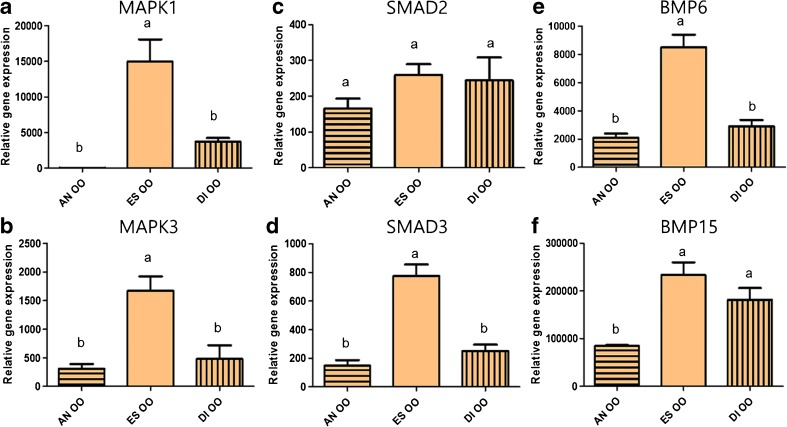
Gene expression of both MAPK1 and MAPK3 in immature oocytes (OOs) was significantly upregulated in the estrus stage (14,960 ± 3,121 and 1,668 ± 253.4, P < 0.05, Fig. 3a, b), similar to SMAD3 (774.6 ± 79.62, P < 0.05, Fig. 3d). However, OOs did not show significant differences in SMAD2 expression among the three stages (Fig. 3c). There was a significantly higher gene expression of BMP6 (8,500 ± 895.4, P < 0.05)in the estrus stage, but no difference was observed between estrus and diestrus stages for BMP15 (Fig. 3e, f).
Fig. 3.
The relative mRNA expression of MAPK1, MAPK3, SMAD2, SMAD3, BMP6, and BMP15 standardized with mRNA expression of beta-actin in oocytes (OOs) during the anestrus, estrus, and diestrus stages. Gene expression of MAPK1 and MAPK3 in OOs during the estrus cycle (a, b). Gene expression of SMAD2 and SMAD3 in OOs during the estrus cycle (c, d). Gene expression of BMP6 and BMP15 in OOs during the estrus cycle (e, f). Different superscripts (a–b) represent significant differences among groups (P < 0.05). AN OO oocytes in anestrus stage, ES OO oocytes in estrus stage, DI OO oocytes in diestrus stage
Effect of co-culture with canine oviduct cells on nuclear maturation of canine oocytes
As shown in Table 2, the GV rate was significantly lower in oocytes co-cultured with canine oviduct cells (32.5 ± 3.1%) compared to that of the control group (70.5 ± 4.7%). The frequency of MI stage in the co-culture group was higher than that in the control group (46.7 ± 7.1 vs. 22.1 ± 4.9%; P < 0.05). Moreover, oocytes matured in co-culture with oviduct cells yielded a higher maturation rate to MII (10.0 ± 1.5%) compared to the control group (3.2 ± 1.4%).
Table 2.
Meiotic status of canine oocytes co-cultured with and without canine oviduct cells
| Group | No. of oocytesa | Number of oocytes (% ± S.E.M.) | |||
|---|---|---|---|---|---|
| GV | GVBD | MI | MII | ||
| Control | 95 | 67 (70.5 ± 4.7)a | 4 (4.2 ± 2.5)a | 21 (22.1 ± 4.9)a | 3 (3.2 ± 1.4)a |
| Oviduct cells | 120 | 39 (32.5 ± 3.1)b | 13 (10.8 ± 2.7)a | 56 (46.7 ± 7.1)b | 12 (10.0 ± 1.5)b |
Within a column, values with different superscripts differ significantly (P < 0.05)
GV germinal vesicle, GVBD germinal vesicle breakdown, MI metaphase I, MII metaphase II
aThree replications were performed
Discussion
Fully grown oocytes within the ovarian follicles are surrounded by specialized granulosa cells, namely cumulus cells, to form COCs [31]. In most mammals, the COCs surrounded by granulosa cells and cumulus cells undergo maturation in pre-ovulatory follicles and ovulation occurs following LH surge. Those granulosa cells and cumulus cells support the oocyte meiotic resumption with the activation of MAPK1/3 and SMAD2/3 signaling pathways. Although canine COCs are surrounded by several layers of cumulus cells, nuclear maturation is completed within the mid-portion of the oviduct. Thus, the oviduct cells could potentially affect oocyte maturation as well as cumulus cells [32]. Here, we hypothesized that genes related to oocyte maturation would be highly expressed in oviduct cells or cumulus cells at the estrus stage compared to other stages, and the cell with high expressed genes would affect oocyte nuclear resumption during IVM of canine COCs. The present study attempted to clarify the gene expression patterns related to oocyte maturation in canine oviduct cells, cumulus cells, and immature oocytes relative to different estrous cycle stages.
Based on the hypothesis, we first analyzed MAPK1/3 in oviduct cells, cumulus cells, and immature oocytes derived from different estrous cycle stages. A number of studies have demonstrated that MAPK plays a necessary role in the regulation of meiotic cell cycle progression in oocytes [33]. MAPK1/3 (also commonly known as ERK1/2) is known to be activated by gonadotropins in granulosa and cumulus cells. The increased activation of MAPK in granulosa and cumulus cells stimulates the synthesis of specific proteins that is capable of inducing oocyte meiotic resumption [34, 35]. In the present study, the highest mRNA expression of MAPK1/3 was shown in OCs and OOs from the estrus stage. However, CCs showed high expression of MAPK1 during the estrus stage, while MAPK3 was highly expressed in the diestrus stage. Thus, we suggested that remarkable expression of MAPK1 and MAPK3 in OCs from the estrus stage could affect the meiotic resumption of canine oocytes.
With this in mind, we next investigated the expression of BMPs which plays an essential role in follicular growth and cumulus expansion [36] in canine OOs, CCs, and OCs from three estrous cycle stages. In particular, BMP6 and BMP15 are oocyte-secreted factors which play a crucial role in regulating ovarian follicular development and mammalian reproduction in general [37]. These factors are involved in the BMP pathway and activate the SMAD signaling pathway through binding with the BMP receptors in COCs. BMP6, part of the TGF-β superfamily, plays a critical role in ovarian function and fertility through bidirectional communication between granulosa cells and oocytes [38]. To date, very few studies have investigated effects of BMP6 on mammalian oocytes and embryos; however, recent research demonstrated that treatment of the culture medium with BMP6 enhanced the maturation rate of oocytes and developmental competence of embryos [39]. We demonstrated that BMP6 expression was markedly increased in CCs and OOs derived from the estrus stage, while OCs showed significantly high gene expression of BMP6 in the diestrus stage. This finding suggested that BMP6 in OCs from the diestrus stage would be involved in developmental competence of embryos rather than oocyte maturation. The BMP15 protein found in oocytes during all stages of folliculogenesis helps follicular growth and expansion of cumulus cells in the ovary [40, 41]. In the present study, BMP15 was expressed in canine oocytes from three estrus cycle stages. Interestingly, OCs showed the highest expression of BMP15 in the estrus stage, while CCs had a marked upregulation of BMP15 in the anestrus stage. Based on the report on pigs, BMP15 which is expressed in granulosa and theca cells as well as oocytes would stimulate the development of pre-ovulatory follicles [42]. Therefore, the present study confirmed that the BMP6/15 was expressed not only from canine oocyte but also from canine oviduct cells and cumulus cells in the estrus stage. We suggested that the high expression of BMP15 in OCs from the estrus stage would support the expansion of canine cumulus cells.
The TGF-β superfamily including BMP6/15 mediated the activation of SMAD families which plays a key role in follicular development and oocyte maturation [43, 44]. Next, we investigated the expression of SMAD2/3 regulated by BMPs in canine OOs , CCs, and OCs from different estrous cycle stages. Recent studies demonstrated that SMAD2/3 activation enhanced cumulus expansion, which protects the oocyte from subsequent mechanical and enzymatic stresses during growth, while deletion of SMAD2/3 causes defective expansion of cumulus cells [45, 46]. In the present study, the mRNA expression of SMAD2/3 was significantly upregulated in OCs and CCs derived from the estrus stage. The OOs showed high mRNA expression of SMAD3 during the estrus stage, while no significant differences were observed in SMAD2 expression among estrous cycle stages. We suggested that the highest expression of SMAD2/3 in OCs from the estrus stage might affect oocyte nuclear maturation by supporting cumulus expansion.
Finally, we found that the oviduct cells from the estrus stage exhibit high gene expression of MAPK1/3, SMAD2/3, and BMP15. Based on these results, we hypothesized that co-culture with oviduct cells from the estrus stage during IVM could affect oocyte nuclear maturation in dogs. Our results achieved a higher meiotic resumption rate to MI (46.7%) and MII (10.0%) stages by co-culturing with canine oviduct cells from the estrus stage compared with those of control groups. In other species, it has been proposed that oocytes co-cultured with oviduct cells yielded a higher number of blastocysts, with higher numbers of blastocyst cells [47]. Also, co-culture with oviduct cells for in vitro fertilization increased the proportion of porcine oocytes reaching the two pronucleus stages and decreased the rate of polyspermy compared with controls [48]. In other canine IVM studies, the use of oviduct cells for co-culture with oocytes increased the nuclear resumption rate (3% on MI/AI/MII rate) [4], while higher maturation rate was achieved in the present study by co-culturing with oviduct cells (56.7% on MI/MII rate) (Fig. 4). We assumed that our study used oviduct cells from the estrus stage, showing upregulated gene expressions of MAPK1/3, SMAD2/3, and BMP15, and this would give a beneficial effect on canine IVM. Moreover, the Transwell co-culture system that we applied in this study produced an environment similar to that in in vivo state by permitting cells to secrete molecules to oocytes and this might improve the canine oocyte maturation.
In conclusion, for the first time to our knowledge, oocyte maturation-related gene expression was analyzed in oviduct cells, cumulus cells, and immature oocytes derived from the three estrous cycle stages. The present study demonstrated that oviduct cells in the estrus stage showed high gene expressions of MAPK1/3, SMAD2/3, and BMP15 related to canine oocyte maturation and improved canine oocyte nuclear maturation during IVM. These outcomes will support a key clue for understanding of the cellular signaling pathways or gene expression that occurs during canine oocyte maturation, and the oviduct cells in the estrus stage could be a useful tool for canine IVM. A further study is needed to determine the relationship between gene expression levels of those genes from canine oviduct cells and oocyte after co-culture.
Electronic supplementary material
(DOCX 941 kb)
Acknowledgments
This research was supported by RDA (#PJ010928032017), Korea IPET (#316002-05-2-SB010), Research Institute for Veterinary Science, the BK21 plus program, and global Ph.D. Fellowship Program through the National Research Foundation of Korea (NRF-20142A1021187).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-017-0910-x) contains supplementary material, which is available to authorized users.
References
- 1.Hashimoto S. Application of in vitro maturation to assisted reproductive technology. J Reprod Dev. 2009;55(1):1–10. doi: 10.1262/jrd.20127. [DOI] [PubMed] [Google Scholar]
- 2.Reynaud K, Fontbonne A, Saint-Dizier M, Thoumire S, Marnier C, Tahir MZ, et al. Folliculogenesis, ovulation and endocrine control of oocytes and embryos in the dog. Reprod Domest Anim. 2012;47(Suppl 6):66–69. doi: 10.1111/rda.12055. [DOI] [PubMed] [Google Scholar]
- 3.Chastant-Maillard S, Viaris de Lesegno C, Chebrout M, Thoumire S, Meylheuc T, Fontbonne A, et al. The canine oocyte: uncommon features of in vivo and in vitro maturation. Reprod Fertil Dev. 2011;23(3):391–402. doi: 10.1071/RD10064. [DOI] [PubMed] [Google Scholar]
- 4.Hewitt DA, England GC. Synthetic oviductal fluid and oviductal cell coculture for canine oocyte maturation in vitro. Anim Reprod Sci. 1999;55(1):63–75. doi: 10.1016/S0378-4320(98)00162-6. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS, Seo YI, Yin XJ, Cho SG, Lee SS, Kim NH, et al. Effect of follicle stimulation hormone and luteinizing hormone on cumulus cell expansion and in vitro nuclear maturation of canine oocytes. Reprod Domest Anim. 2007;42(6):561–565. doi: 10.1111/j.1439-0531.2006.00818.x. [DOI] [PubMed] [Google Scholar]
- 6.Bolamba D, Russ KD, Harper SA, Sandler JL, Durrant BS. Effects of epidermal growth factor and hormones on granulosa expansion and nuclear maturation of dog oocytes in vitro. Theriogenology. 2006;65(6):1037–1047. doi: 10.1016/j.theriogenology.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 7.Cui XS, Jin YX, Shen XH, Lee JY, Lee HS, Yin XJ, et al. Epidermal growth factor enhances meiotic resumption of canine oocytes in the presence of BSA. Theriogenology. 2006;66(2):267–274. doi: 10.1016/j.theriogenology.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Hatoya S, Sugiyama Y, Torii R, Wijewardana V, Kumagai D, Sugiura K, et al. Effect of co-culturing with embryonic fibroblasts on IVM, IVF and IVC of canine oocytes. Theriogenology. 2006;66(5):1083–1090. doi: 10.1016/j.theriogenology.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Ghani MA, Shimizu T, Asano T, Suzuki H. In vitro maturation of canine oocytes co-cultured with bovine and canine granulosa cell monolayers. Theriogenology. 2012;77(2):347–355. doi: 10.1016/j.theriogenology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Boruszewska D, Sinderewicz E, Kowalczyk-Zieba I, Grycmacher K, Woclawek-Potocka I. The effect of lysophosphatidic acid during in vitro maturation of bovine cumulus-oocyte complexes: cumulus expansion, glucose metabolism and expression of genes involved in the ovulatory cascade, oocyte and blastocyst competence. Reprod Biol Endocrinol. 2015;13:44. doi: 10.1186/s12958-015-0044-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero-Aguirregomezcorta J, Santa AP, Garcia-Vazquez FA, Coy P, Matas C. Nitric oxide synthase (NOS) inhibition during porcine in vitro maturation modifies oocyte protein S-nitrosylation and in vitro fertilization. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0115044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zavareh S, Karimi I, Salehnia M, Rahnama A. Effect of in vitro maturation technique and alpha lipoic acid supplementation on oocyte maturation rate: focus on oxidative status of oocytes. Int J Fertil Steril. 2016;9(4):442–451. doi: 10.22074/ijfs.2015.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motola S, Cao X, Popliker M, Tsafriri A. Involvement of mitogen-activated protein kinase (MAPK) pathway in LH- and meiosis-activating sterol (MAS)-induced maturation in rat and mouse oocytes. Mol Reprod Dev. 2008;75(10):1533–1541. doi: 10.1002/mrd.20899. [DOI] [PubMed] [Google Scholar]
- 14.Zhang YL, Liu XM, Ji SY, Sha QQ, Zhang J, Fan HY. ERK1/2 activities are dispensable for oocyte growth but are required for meiotic maturation and pronuclear formation in mouse. J Genet Genomics. 2015;42(9):477–485. doi: 10.1016/j.jgg.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Yeo CX, Gilchrist RB, Lane M. Disruption of bidirectional oocyte-cumulus paracrine signaling during in vitro maturation reduces subsequent mouse oocyte developmental competence. Biol Reprod. 2009;80(5):1072–1080. doi: 10.1095/biolreprod.108.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussein TS, Froiland DA, Amato F, Thompson JG, Gilchrist RB. Oocytes prevent cumulus cell apoptosis by maintaining a morphogenic paracrine gradient of bone morphogenetic proteins. J Cell Sci. 2005;118(Pt 22):5257–5268. doi: 10.1242/jcs.02644. [DOI] [PubMed] [Google Scholar]
- 17.Glister C, Kemp CF, Knight PG. Bone morphogenetic protein (BMP) ligands and receptors in bovine ovarian follicle cells: actions of BMP-4, -6 and -7 on granulosa cells and differential modulation of Smad-1 phosphorylation by follistatin. Reproduction. 2004;127(2):239–254. doi: 10.1530/rep.1.00090. [DOI] [PubMed] [Google Scholar]
- 18.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9(9):726–735. [PubMed] [Google Scholar]
- 19.Concannon PW. Reproductive cycles of the domestic bitch. Anim Reprod Sci. 2011;124(3–4):200–210. doi: 10.1016/j.anireprosci.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 20.Kim MJ, Oh HJ, Kim GA, Jo YK, Choi J, Lee BC. Application of chemiluminescence enzyme immunoassay method to collect in vivo matured oocyte in dog cloning. J Vet Clin. 2014;31(4):267–271. doi: 10.17555/ksvc.2014.08.31.4.267. [DOI] [Google Scholar]
- 21.Schutte AP. Canine vaginal cytology. I. Technique and cytological morphology. J Small Anim Pract. 1967;8(6):301–306. doi: 10.1111/j.1748-5827.1967.tb04554.x. [DOI] [PubMed] [Google Scholar]
- 22.van Goethem B, Schaefers-Okkens A, Kirpensteijn J. Making a rational choice between ovariectomy and ovariohysterectomy in the dog: a discussion of the benefits of either technique. Vet Surg. 2006;35(2):136–143. doi: 10.1111/j.1532-950X.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 23.Oh HJ, Fibrianto YH, Kim MK, Jang G, Hossein MS, Kim HJ, et al. Effects of canine serum collected from dogs at different estrous cycle stages on in vitro nuclear maturation of canine oocytes. Zygote. 2005;13(3):227–232. doi: 10.1017/S0967199405003242. [DOI] [PubMed] [Google Scholar]
- 24.Kim MK, Fibrianto YH, Oh HJ, Jang G, Kim HJ, Lee KS, et al. Effects of estradiol-17beta and progesterone supplementation on in vitro nuclear maturation of canine oocytes. Theriogenology. 2005;63(5):1342–1353. doi: 10.1016/j.theriogenology.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Kim GA, Oh HJ, Lee TH, Lee JH, Oh SH, Lee JH, et al. Effect of culture medium type on canine adipose-derived mesenchymal stem cells and developmental competence of interspecies cloned embryos. Theriogenology. 2014;81(2):243–249. doi: 10.1016/j.theriogenology.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 26.Lee BC, Kim MK, Jang G, Oh HJ, Yuda F, Kim HJ, et al. Dogs cloned from adult somatic cells. Nature. 2005;436(7051):641. doi: 10.1038/436641a. [DOI] [PubMed] [Google Scholar]
- 27.Hong SG, Oh HJ, Park JE, Kim MJ, Kim GA, Park EJ, et al. Production of offspring from cloned transgenic RFP female dogs and stable generational transmission of the RFP gene. Genesis. 2011;49(11):835–840. doi: 10.1002/dvg.20772. [DOI] [PubMed] [Google Scholar]
- 28.Kim MJ, Oh HJ, Park JE, Kim GA, Hong SG, Jang G, et al. Generation of transgenic dogs that conditionally express green fluorescent protein. Genesis. 2011;49(6):472–478. doi: 10.1002/dvg.20737. [DOI] [PubMed] [Google Scholar]
- 29.Saadeldin IM, Elsayed A, Kim SJ, Moon JH, Lee BC. A spatial model showing differences between juxtacrine and paracrine mutual oocyte-granulosa cells interactions. Indian J Exp Biol. 2015;53(2):75–81. [PubMed] [Google Scholar]
- 30.de Avila Rodrigues B, Rodrigues JL. Influence of reproductive status on in vitro oocyte maturation in dogs. Theriogenology. 2003;60(1):59–66. doi: 10.1016/S0093-691X(02)01301-8. [DOI] [PubMed] [Google Scholar]
- 31.Kimura N, Hoshino Y, Totsukawa K, Sato E. Cellular and molecular events during oocyte maturation in mammals: molecules of cumulus-oocyte complex matrix and signalling pathways regulating meiotic progression. Soc Reprod Fertil Suppl. 2007;63:327–342. [PubMed] [Google Scholar]
- 32.Luvoni GC, Chigioni S, Allievi E, Macis D. Factors involved in vivo and in vitro maturation of canine oocytes. Theriogenology. 2005;63(1):41–59. doi: 10.1016/j.theriogenology.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Liang CG, Su YQ, Fan HY, Schatten H, Sun QY. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21(9):2037–2055. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- 34.Ebeling S, Schuon C, Meinecke B. Mitogen-activated protein kinase phosphorylation patterns in pig oocytes and cumulus cells during gonadotrophin-induced resumption of meiosis in vitro. Zygote. 2007;15(2):139–147. doi: 10.1017/S0967199406004011. [DOI] [PubMed] [Google Scholar]
- 35.Fan HY, Huo LJ, Chen DY, Schatten H, Sun QY. Protein kinase C and mitogen-activated protein kinase cascade in mouse cumulus cells: cross talk and effect on meiotic resumption of oocyte. Biol Reprod. 2004;70(4):1178–1187. doi: 10.1095/biolreprod.103.024737. [DOI] [PubMed] [Google Scholar]
- 36.Fatehi AN, van den Hurk R, Colenbrander B, Daemen AJ, van Tol HT, Monteiro RM, et al. Expression of bone morphogenetic protein2 (BMP2), BMP4 and BMP receptors in the bovine ovary but absence of effects of BMP2 and BMP4 during IVM on bovine oocyte nuclear maturation and subsequent embryo development. Theriogenology. 2005;63(3):872–889. doi: 10.1016/j.theriogenology.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132(2):191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- 38.Buccione R, Schroeder AC, Eppig JJ. Interactions between somatic cells and germ cells throughout mammalian oogenesis. Biol Reprod. 1990;43(4):543–547. doi: 10.1095/biolreprod43.4.543. [DOI] [PubMed] [Google Scholar]
- 39.Pan Y, He H, Cui Y, Baloch AR, Li Q, Fan J, et al. Recombinant human bone morphogenetic protein 6 enhances oocyte reprogramming potential and subsequent development of the cloned yak embryos. Cell Reprogram. 2015;17(6):484–493. doi: 10.1089/cell.2015.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su YQ, Sugiura K, Wigglesworth K, O’Brien MJ, Affourtit JP, Pangas SA, et al. Oocyte regulation of metabolic cooperativity between mouse cumulus cells and oocytes: BMP15 and GDF9 control cholesterol biosynthesis in cumulus cells. Development. 2008;135(1):111–121. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 41.Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12(12):1809–1817. doi: 10.1210/mend.12.12.0206. [DOI] [PubMed] [Google Scholar]
- 42.Paradis F, Novak S, Murdoch GK, Dyck MK, Dixon WT, Foxcroft GR. Temporal regulation of BMP2, BMP6, BMP15, GDF9, BMPR1A, BMPR1B, BMPR2 and TGFBR1 mRNA expression in the oocyte, granulosa and theca cells of developing preovulatory follicles in the pig. Reproduction. 2009;138(1):115–129. doi: 10.1530/REP-08-0538. [DOI] [PubMed] [Google Scholar]
- 43.Kuo FT, Fan K, Ambartsumyan G, Menon P, Ketefian A, Bentsi-Barnes IK, et al. Relative expression of genes encoding SMAD signal transduction factors in human granulosa cells is correlated with oocyte quality. J Assist Reprod Genet. 2011;28(10):931–938. doi: 10.1007/s10815-011-9609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimasaki S, Moore RK, Otsuka F, Erickson GF. The bone morphogenetic protein system in mammalian reproduction. Endocr Rev. 2004;25(1):72–101. doi: 10.1210/er.2003-0007. [DOI] [PubMed] [Google Scholar]
- 45.Li Q, Pangas SA, Jorgez CJ, Graff JM, Weinstein M, Matzuk MM. Redundant roles of SMAD2 and SMAD3 in ovarian granulosa cells in vivo. Mol Cell Biol. 2008;28(23):7001–7011. doi: 10.1128/MCB.00732-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dragovic RA, Ritter LJ, Schulz SJ, Amato F, Thompson JG, Armstrong DT, et al. Oocyte-secreted factor activation of SMAD 2/3 signaling enables initiation of mouse cumulus cell expansion. Biol Reprod. 2007;76(5):848–857. doi: 10.1095/biolreprod.106.057471. [DOI] [PubMed] [Google Scholar]
- 47.Kidson A, Schoevers E, Langendijk P, Verheijden J, Colenbrander B, Bevers M. The effect of oviductal epithelial cell co-culture during in vitro maturation on sow oocyte morphology, fertilization and embryo development. Theriogenology. 2003;59(9):1889–1903. doi: 10.1016/S0093-691X(02)01291-8. [DOI] [PubMed] [Google Scholar]
- 48.Bureau M, Bailey JL, Sirard MA. Influence of oviductal cells and conditioned medium on porcine gametes. Zygote. 2000;8(2):139–144. doi: 10.1017/S0967199400000915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 941 kb)