Figure 1.
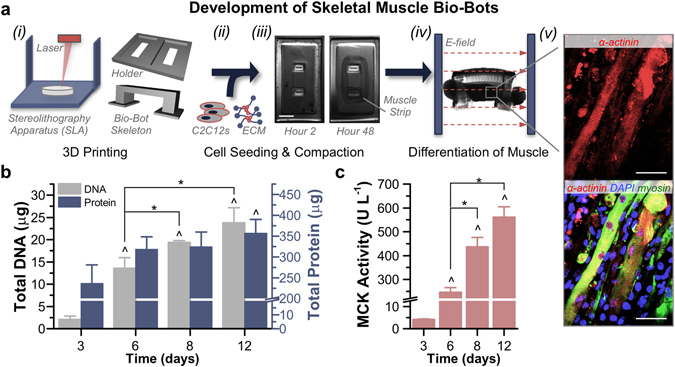
Development and Differentiation of Skeletal Muscle Bio-Bots. (a) Modular bio-bots were assembled and differentiated in a stepwise manner. (i) A Stereolithography apparatus (SLA) was used to 3D print a millimeter-scale hydrogel skeleton and holder. (ii) C2C12s were mixed with a liquid solution of ECM proteins that included fibrinogen, thrombin and Matrigel. (iii) When added to the holder, the cell-gel solution compacted to form a solid muscle strip. Scale bar, 2 mm. (iv) The freestanding bio-bot (consisting of the muscle strip coupled to the hydrogel skeleton) could be released from the holder and subjected to electrical or optical stimulation. (v) Immunostaining revealed the presence of striated and multinucleated myotubes (α-actinin, red; MF-20 myosin, green; DAPI nuclear stain, blue). Scale bar, 50 μm. (b) Total DNA and protein levels increased over the time course of the experiment (n = 3–4 muscle strips per time point). There was no statistically significant difference in protein/DNA ratios between days 8 (17 ± 2.8 μg protein μg DNA−1) and 12 (15.7 ± 2.5 μg protein μg DNA−1). (c) Muscle creatine kinase (MCK) activity was significantly increased as early as day 6 and reached a maximum output on day 12 (n = 3 muscle strips per time point). The rate of increased MCK activity slowed between days 8 and 12, confirming that this was a relevant stopping point for the experiments. All plots represent mean ± SEM. * indicates significance (p < 0.05) between conditions at the same time point; ^ indicates significance compared to initial time point.
