Abstract
Background
A previous Phase IIIb study (NCT01462929) in patients with moderate to severe COPD demonstrated that 6 weeks of treatment with aclidinium led to improvements in 24-hour bronchodilation comparable to those with tiotropium, and improvement of symptoms versus placebo. This post hoc analysis was performed to assess the effect of treatment in the symptomatic patient group participating in the study.
Methods
Symptomatic patients (defined as those with Evaluating Respiratory Symptoms [E-RS™] in COPD baseline score ≥10 units) received aclidinium bromide 400 μg twice daily (BID), tiotropium 18 μg once daily (QD), or placebo, for 6 weeks. Lung function, COPD respiratory symptoms, and incidence of adverse events (AEs) were assessed.
Results
In all, 277 symptomatic patients were included in this post hoc analysis. Aclidinium and tiotropium treatment improved forced expiratory volume in 1 second (FEV1) from baseline to week 6 at all time points over 24 hours versus placebo. In addition, improvements in FEV1 from baseline during the nighttime period were observed for aclidinium versus tiotropium on day 1 (aclidinium 157 mL, tiotropium 67 mL; P<0.001) and week 6 (aclidinium 153 mL, tiotropium 90 mL; P<0.05). Aclidinium improved trough FEV1 from baseline versus placebo and tiotropium at day 1 (aclidinium 136 mL, tiotropium 68 mL; P<0.05) and week 6 (aclidinium 137 mL, tiotropium 71 mL; P<0.05). Aclidinium also improved early-morning and nighttime symptom severity, limitation of early-morning activities, and E-RS Total and domain scores versus tiotropium (except E-RS Chest Symptoms) and placebo over 6 weeks. Tolerability showed similar incidence of AEs in each arm.
Conclusion
In this post hoc analysis of symptomatic patients with moderate to severe COPD, aclidinium 400 μg BID provided additional improvements compared with tiotropium 18 μg QD in: 1) bronchodilation, particularly during the nighttime, 2) daily COPD symptoms (E-RS), 3) early-morning and nighttime symptoms, and 4) early-morning limitation of activity.
Keywords: COPD, 24-hour bronchodilation, long-acting muscarinic antagonist, nighttime, symptoms
Introduction
Symptoms of COPD can vary in severity over a 24-hour period, and studies indicate that they are generally worse in the early morning and at nighttime.1–3 Symptoms include chronic cough, sputum production, and breathlessness, which can severely impact on a patient’s daily activities and overall well-being,3 and have a corresponding high socioeconomic burden.4 Estimates suggest that the frequency of nocturnal symptoms and symptomatic sleep disturbance may exceed 75% in patients with COPD, and potential long-term consequences may include lung function changes, increased exacerbation frequency, emergence or worsening of cardiovascular disease, impaired quality of life, and increased mortality.1 It is therefore important that symptoms over the entire 24-hour day are identified and managed appropriately.
In order to provide appropriate therapy, clinical guidelines (Global initiative for chronic Obstructive Lung Disease [GOLD]) suggest that symptoms, airflow limitation, and risk of exacerbations are assessed.5 Patients are classified into one of four groups according to their symptom burden and risk of exacerbations: A, low risk, less symptoms; B, low risk, more symptoms; C, high risk, less symptoms; or D, high risk, more symptoms;5 current evidence suggests that bronchodilator treatment may be more effective in those patients who are considered symptomatic (ie, groups B and D).5
Bronchodilator therapies are a mainstay of COPD treatment, with two classes of long-acting bronchodilators currently available: long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs). LAMAs inhibit the action of acetylcholine at muscarinic receptors, while LABAs enhance cAMP signaling through stimulation of β2-adrenergic receptors, resulting in the relaxation of bronchial smooth muscle.5 The LAMA aclidinium bromide is a maintenance bronchodilator therapy for adults with COPD.
The efficacy and tolerability results from a Phase IIIb study in patients with moderate to severe COPD, who received either aclidinium 400 μg twice daily (BID), the active comparator tiotropium 18 μg once daily (QD), or placebo have been previously reported.6 Briefly, following 6 weeks of treatment, patients receiving aclidinium 400 μg BID demonstrated improvements in 24-hour bronchodilation, compared with placebo, that were comparable with tiotropium 18 μg QD. In addition, COPD symptoms significantly improved from baseline with aclidinium, but not tiotropium, compared with placebo.6 These results were similar to those observed in a prior 2-week Phase IIa trial.7 Furthermore, a recent real-world study in patients with COPD reported improvements in nighttime and early-morning symptoms, limitation of morning activities, and quality of life over 3 months with aclidinium 400 μg BID, compared with baseline.8 Since aclidinium has a greater impact on COPD symptoms than tiotropium,9,10 and the “more symptomatic” patient groups stand to benefit more from bronchodilator treatment than the “less symptomatic” groups, aclidinium may provide an additional therapeutic benefit over tiotropium in these patients.
This study reports the findings of a post hoc analysis, which focused on the response in the symptomatic patient group. The key objective of this analysis was to identify any differences in 24-hour lung function and symptom control between treatment with aclidinium 400 μg BID and tiotropium 18 μg QD in this population of patients.
Methods
Study design and patients
Overall study
This was a randomized, double-blind, double-dummy, placebo- and active comparator-controlled, multicenter Phase IIIb study in patients with moderate to severe COPD (ClinicalTrials.gov identifier: NCT01462929). Full details of the study design and inclusion/exclusion criteria have been published previously.6 Briefly, patients with COPD aged ≥40 years with a smoking history (current or previous) of ≥10 pack-years were eligible to enter the study. Patients with moderate to severe COPD (for whom long-acting bronchodilators are recommended)5 had post-salbutamol forced expiratory volume in 1 second (FEV1) ≥30% and <80% of the predicted normal value, FEV1/forced vital capacity <70%. Use of long-acting bronchodilators other than the investigative treatment was not permitted. Use of salbutamol pressurized metered dose inhaler (100 μg/puff) was permitted as relief medication as needed (except ≤6 hours before each visit). Patients were permitted to continue use of oral sustained-release theophylline (use of other methylxanthines was not permitted), inhaled corticosteroids, and oral or parenteral corticosteroids (equivalent to ≤10 mg/day or 20 mg every other day of prednisone) if treatment was stable ≥4 weeks prior to screening, except ≤6 hours before each visit. Oxygen therapy (except ≤2 hours before each visit) was permitted. After a screening visit, patients underwent a 2- to 3-week run-in period to assess disease stability. Eligible patients were randomized (2:2:1) to receive aclidinium bromide 400 μg BID in the morning and evening via the Genuair™/Pressair® (registered trademark of AstraZeneca group of companies; for use within the USA as Pressair® and as Genuair™ within all other licensed territories) multidose dry powder inhaler, tiotropium 18 μg QD in the morning via the HandiHaler®, or placebo for 6 weeks.
The study was approved by an independent ethics committee at each site (Table S1) and was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonisation, and Good Clinical Practice guidelines. All patients provided written informed consent.
Post hoc analysis
This post hoc analysis assessed symptomatic patients, defined as those patients with an Evaluating Respiratory Symptoms in COPD (E-RS:COPD™ [The EXACT™ and E-RS™ are owned by Evidera. Permission to use these instruments may be obtained from Evidera {exactpro@evidera.com}]; formerly known as EXAcerbations of Chronic pulmonary disease Tool [EXACT]-RS) baseline score ≥10 units. This threshold was chosen based on data indicating that an E-RS score ≥10 units differentiated between asymptomatic (GOLD groups A and C) and symptomatic (GOLD groups B and D) patients.11
Assessments and endpoints
Lung function
Lung function was assessed over 24 hours post-dose on day 1 and at week 6. The primary endpoint was change from baseline in normalized FEV1 area under the curve (AUC) over 24 hours post-morning dose (AUC0–24/24 h) at week 6. The secondary endpoint was change from baseline in normalized FEV1 AUC over the nighttime period (AUC12–24/12 h) at week 6. An additional lung function endpoint was change from baseline in morning pre-dose (trough) FEV1.
COPD symptoms
Every evening, patients completed the 14-item EXACT (recall period of “today”) via electronic diaries and daily COPD symptoms scores were derived using E-RS scoring algorithms. The E-RS uses the 11 respiratory symptom items from the 14-item EXACT and assesses both overall daily respiratory COPD symptoms (RS-Total score; score range, 0–40, with higher scores indicating more severe symptoms) and specific respiratory symptoms using three subscales (RS-Breathlessness [score range, 0–17], RS-Cough and Sputum [score range, 0–11], and RS-Chest Symptoms [the sum of three items related to chest congestion/discomfort; score range, 0–12]).12,13 E-RS Total and domain scores were assessed at baseline and over the 6-week study duration. Patients who achieved a clinically meaningful improvement from baseline (E-RS Total score ≥−2 units) were considered to be responders; this responder definition was proposed based on results from three randomized controlled trials.14 Responder status was assessed over the 6 weeks of the study.
To assess the severity of early-morning and nighttime symptoms, an additional COPD symptoms questionnaire developed by the study sponsor was completed by patients each morning via electronic diaries (5-point scale: 1= “did not experience symptoms”; 5= “very severe”) and included individual morning symptoms of cough, wheeze, shortness of breath, and phlegm (5-point scale: 0= “no symptoms”; 4= “very severe symptoms”), as well as limitation of morning activities (5-point scale: 1= “not at all”; 5= “a very great deal”). Since this study, these questionnaires have been developed and evaluated further.15,16
Safety and tolerability
Treatment-emergent adverse events (TEAEs) were recorded throughout the study.
Statistical analyses
Efficacy data are reported for the intent-to-treat population, defined as all randomized patients who received at least one dose of study medication and who had at least one baseline and post-baseline FEV1 value. Endpoints were assessed using an analysis of covariance model with treatment and sex as factors, and age and baseline values as covariates. Between-group least squares mean differences and 95% confidence intervals were calculated for all treatment group comparisons.
Results
Patients
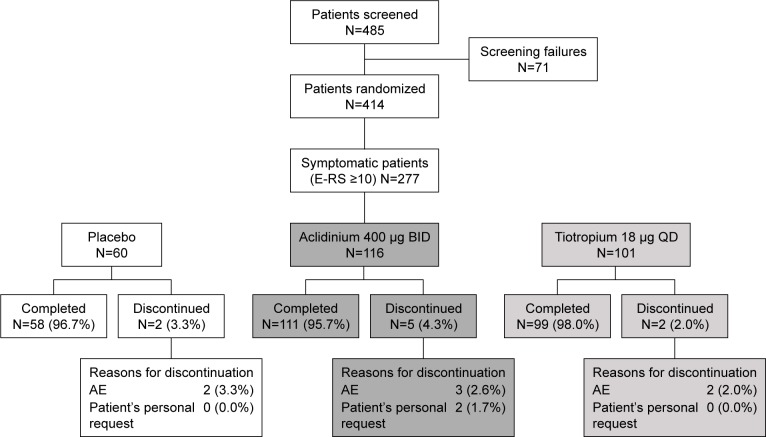
In all, 414 patients were randomized to treatment in the overall study (2:2:1 ratio), of which 277 were defined as symptomatic (E-RS baseline score ≥10 units) and included in this post hoc subgroup analysis (placebo: n=60; aclidinium 400 μg: n=116; tiotropium 18 μg: n=101) (Figure 1). The percentages of patients in each treatment arm of this post hoc analysis were similar to those in the primary study (placebo, 21.7% vs 20.5%; aclidinium 400 μg, 41.9% vs 41.3%; tiotropium 18 μg, 36.5% vs 38.2%, respectively).
Figure 1.
Patient flow diagram.
Abbreviations: AE, adverse event; BID, twice daily; E-RS, Evaluating Respiratory Symptoms; QD, once daily.
Demographics and baseline characteristics in the subgroup of symptomatic patients were similar to those in the overall study population (symptomatic patients: mean age 62.1 years, 65.0% male, 54.5% current smokers, post-bronchodilator FEV1 54.6% predicted). Patient demographics and baseline characteristics for symptomatic patients were also similar across treatment arms, with the exception of a higher proportion of male patients in the active treatment groups compared with placebo, and a higher proportion of patients with severe COPD in the tiotropium group (Table 1). Mean post-bronchodilator percent predicted FEV1 and COPD symptoms scores at baseline were similar across treatment arms (Table 1).
Table 1.
Patient demographics in symptomatic patients (E-RS baseline score ≥10 units)
| Placebo (N=60) | Aclidinium 400 µg BID (N=116) | Tiotropium 18 µg QD (N=101) | |
|---|---|---|---|
| Gender (male), n (%) | 35 (58.3) | 75 (64.7) | 70 (69.3) |
| Age (years), mean (SD) | 62.1 (8.2) | 61.4 (8.4) | 62.9 (8.1) |
| Race, n (%) | |||
| White | 59 (98.3) | 116 (100) | 101 (100) |
| BMI (kg/m2), mean (SD) | 26.8 (5.4) | 27.4 (4.9) | 27.1 (4.8) |
| Current smoker, n (%) | 31 (51.7) | 64 (55.2) | 56 (55.5) |
| Smoking consumption (pack-years), mean (SD) | 38.8 (14.3) | 41.7 (23.7) | 42.4 (17.1) |
| COPD duration (years), mean (SD) | 9.63 (6.7) | 8.44 (5.8) | 8.44 (6.6) |
| Post-bronchodilator FEV1 (L) | |||
| Mean (SD) | 1.56 (0.54) | 1.59 (0.50) | 1.53 (0.49) |
| % predicted, mean (SD) | 55.2 (12.1) | 55.5 (13.4) | 53.0 (13.1) |
| Mean bronchial reversibility, % (SD) | 10.1 (9.7) | 14.7 (15.2) | 10.8 (12.8) |
| COPD severity,a n (%) | |||
| Moderate | 40 (66.7) | 72 (62.1) | 56 (56.0) |
| Severe | 20 (33.3) | 44 (37.9) | 44 (44.0) |
| Exacerbations in previous year, n (%) | |||
| 0 | 46 (76.7) | 75 (64.7) | 67 (66.3) |
| 1 | 12 (20.0) | 31 (26.7) | 30 (29.7) |
| ≥2 | 2 (3.3) | 10 (8.6) | 4 (4.0) |
| E-RS Total score (SD) | 16.2 (4.3) | 16.4 (4.2) | 15.8 (3.8) |
| E-RS Cough and Sputum (SD) | 4.3 (1.6) | 4.5 (1.4) | 4.4 (1.1) |
| E-RS Chest Symptoms (SD) | 3.9 (1.3) | 4.0 (1.4) | 3.7 (1.3) |
| E-RS Breathlessness (SD) | 8.0 (2.5) | 7.9 (2.5) | 7.7 (2.5) |
| Severity of early-morning symptoms | |||
| Any (SD) | 2.5 (0.5) | 2.6 (0.5) | 2.5 (0.5) |
| Cough (SD) | 1.4 (0.8) | 1.5 (0.7) | 1.4 (0.7) |
| Wheeze (SD) | 0.9 (0.9) | 0.9 (0.8) | 0.8 (0.7) |
| Shortness of breath (SD) | 1.3 (0.7) | 1.3 (0.8) | 1.1 (0.8) |
| Phlegm (SD) | 0.8 (0.9) | 1.1 (0.9) | 0.9 (0.9) |
| Limitation of activity (SD) | 2.3 (0.6) | 2.3 (0.6) | 2.3 (0.7) |
| Severity of nighttime symptoms (SD) | 2.1 (0.7) | 2.2 (0.6) | 2.1 (0.6) |
Notes:
GOLD stage II (moderate): FEV1/FVC <0.70, and post-bronchodilator FEV1 ≥50% and <80% predicted; GOLD stage III (severe): FEV1/FVC <0.70, and post-bronchodilator FEV1 ≥30% and <50% predicted.
Abbreviations: BID, twice daily; BMI, body mass index; E-RS, Evaluating Respiratory Symptoms; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; QD, once daily; SD, standard deviation.
Efficacy
Lung function
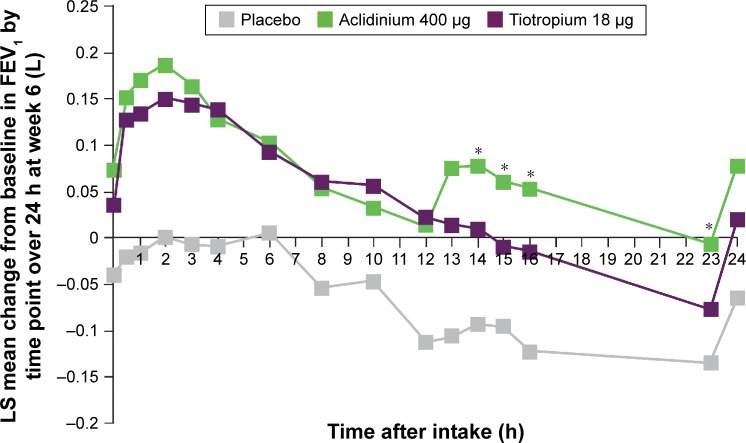
Lung function endpoints in the subgroup of symptomatic patients were similar to those in the overall population. Aclidinium 400 μg BID and tiotropium 18 μg QD both improved FEV1 over 24 hours (AUC0–24/24 h) from baseline at week 6 compared with placebo (aclidinium, 140 mL; tiotropium, 106 mL; both P<0.01). Furthermore, treatment with aclidinium 400 μg BID and tiotropium 18 μg QD improved FEV1 from baseline at week 6 at all time points over 24 hours, compared with placebo (Figure 2). During the nighttime period (AUC12–24 h/12 h), improvements from baseline compared with placebo were greater with aclidinium 400 μg BID than tiotropium 18 μg QD on day 1 (157 vs 67 mL for aclidinium and tiotropium, respectively; P<0.001) and week 6 (153 vs 90 mL for aclidinium and tiotropium, respectively; P<0.05).
Figure 2.
Symptomatic patients: mean changes from baseline in FEV1 at week 6 over 24 hours.
Notes: Placebo N=60, aclidinium 400 μg N=116, tiotropium 18 μg N=101. *P<0.05 versus tiotropium; P<0.05 for aclidinium 400 μg versus placebo and tiotropium 18 μg versus placebo at all time points, except aclidinium at 10 hours (P=0.08) and tiotropium at 23 hours (P=0.14).
Abbreviations: FEV1, forced expiratory volume in 1 second; LS, least squares.
Aclidinium 400 μg BID also demonstrated improvements in trough FEV1 from baseline versus placebo and tiotropium at day 1 (136 vs 68 mL for aclidinium and tiotro-pium, respectively; P<0.05) and week 6 (137 vs 71 mL for aclidinium and tiotropium, respectively; P<0.05) in symptomatic patients (Figure 3).
Figure 3.
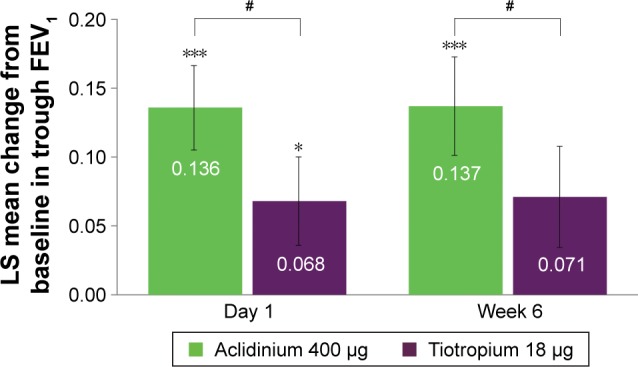
Symptomatic patients: change from baseline in trough FEV1 versus placebo at day 1 and week 6.
Notes: Placebo N=60, aclidinium 400 μg N=116, tiotropium 18 μg N=101. *P<0.05, ***P<0.001 versus placebo; #P<0.05 versus tiotropium.
Abbreviations: FEV1, forced expiratory volume in 1 second; LS, least squares.
COPD symptoms in symptomatic patients
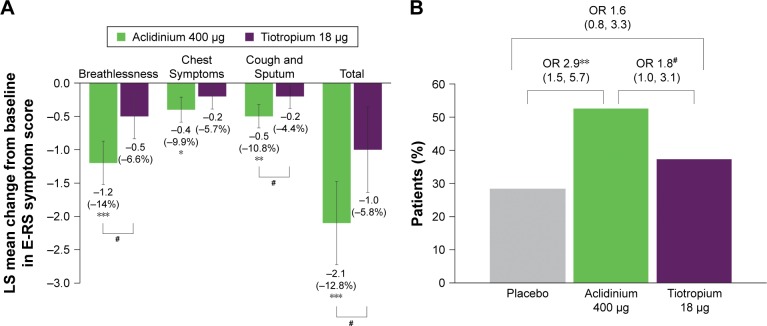
In this subgroup of symptomatic patients, the improvement from baseline in E-RS Total score was greater with aclidinium compared with placebo (P<0.001) and tiotropium (P<0.05) over 6 weeks (Figure 4A): −2.8 units with aclidinium versus −0.7 units with placebo and −1.6 units with tiotropium. For each of the E-RS domains, greater improvements from baseline in E-RS score in symptomatic patients were also observed for aclidinium over 6 weeks of treatment (RS-Breathlessness and RS-Cough and Sputum: P<0.05 vs tiotropium and P<0.01 vs placebo; RS-Chest Symptoms: P<0.05 vs placebo) (Figure 4A). A higher percentage of patients in the aclidinium 400 μg treatment arm were E-RS responders (52.6%) compared with placebo (28.3%; P<0.01) and tiotropium 18 μg (37.6%; P<0.05) (Figure 4B) over 6 weeks.
Figure 4.
Symptomatic patients: change from baseline in E-RS Total and domain symptom scores versus placebo (A) and percentage of E-RS responders (B) at week 6.
Notes: Percentage reduction is shown in brackets. Placebo N=60, aclidinium 400 μg N=116, tiotropium 18 μg N=101. *P<0.05, **P<0.01, ***P<0.001 versus placebo; #P<0.05 versus tiotropium. Scores: 0–40 for Total, 0–17 for Breathlessness, 0–12 for Chest Symptoms, 0–11 for Cough and Sputum.
Abbreviations: E-RS, Evaluating Respiratory Symptoms; LS, least squares; OR, odds ratio.
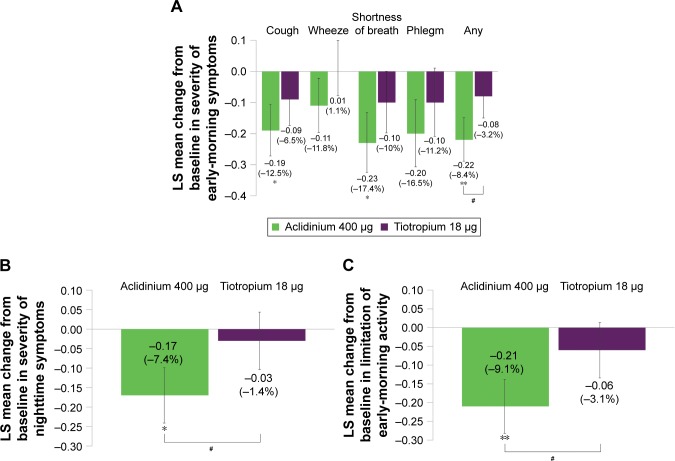
Overall early-morning symptom severity was reduced in the subgroup of symptomatic patients over 6 weeks with aclidinium treatment versus placebo (P<0.01) and tiotropium (P<0.05; Figure 5A). Aclidinium also demonstrated improvements in individual early-morning symptom domains; shortness of breath and cough symptom scores improved in symptomatic patients treated with aclidinium compared with placebo over 6 weeks (both P<0.05; Figure 5A). A reduction in overall nighttime symptom severity from baseline was observed over 6 weeks with aclidinium versus placebo and tiotropium in symptomatic patients (both P<0.05; Figure 5B). Numerical improvements in early-morning or nighttime symptom severity were observed for tiotropium versus placebo in this subgroup. In symptomatic patients, limitation of early-morning activity caused by COPD symptoms was reduced from baseline over 6 weeks with aclidinium versus placebo (P<0.01) and tiotropium (P<0.05), but not with tiotropium versus placebo (Figure 5C).
Figure 5.
Symptomatic patients: change from baseline in symptom severity in the early morning (A) and nighttime (B), and limitation of early-morning activity (C) over 6 weeks.
Notes: Percentage reduction is shown in brackets. Placebo N=60, aclidinium 400 μg N=116, tiotropium 18 μg N=101. *P<0.05, **P<0.01 versus placebo; #P<0.05 versus tiotropium. Specific symptoms (A): 1= mild, 2= moderate, 3= severe, 4= very severe; any symptom: 1= no symptoms, 2= mild, 3= moderate, 4= severe, 5= very severe. For patients with no symptom, a zero value was assigned. Nighttime symptom severity (B): 1= mild, 2= moderate, 3= severe, 4= very severe. Limitation of activity (C): 1= not at all, 2= a little, 3= moderately, 4= a good deal, 5= a very good deal.
Abbreviation: LS, least squares.
Safety and tolerability
In the subgroup of symptomatic patients, the incidence of TEAEs was comparable in the placebo (26.7%), aclidinium (28.4%), and tiotropium (32.7%) groups. Similar to the overall study population, the most commonly reported TEAEs in symptomatic patients were headache (5.8%) and nasopharyngitis (5.1%). Other common TEAEs (≥2% of patients overall) were COPD exacerbation (2.5%), back pain (2.5%), and cough (2.2%). The majority of TEAEs were mild or moderate in intensity. There were few serious TEAEs (1.4% overall) and no deaths in the subgroup of symptomatic patients. In total, five patients (1.8%) discontinued due to TEAEs and one patient (0.4%) discontinued due to a serious TEAE, with COPD exacerbation being the most common cause (1.4%).
Discussion
Assessment of treatment efficacy in symptomatic patients has clinical significance, as treatment guidelines recommend that such patients are treated in order to improve lung function and reduce symptoms.17 This post hoc analysis was performed to evaluate the 24-hour effect of treatment with aclidinium bromide 400 μg BID, tiotropium 18 μg QD, or placebo, in 277 symptomatic patients with moderate to severe COPD participating in a Phase III study. This subgroup of symptomatic patients constituted a substantial proportion of the overall patients (277/414; 45%).
In the subgroup of symptomatic patients, 6 weeks of treatment with aclidinium BID showed improvements from baseline in FEV1 at all time points over 24 hours (compared with placebo), and FEV1 was higher than tiotropium QD at most 12- to 24-hour time points. Aclidinium treatment also led to greater improvements in trough FEV1 compared with tiotropium. Furthermore, during the nighttime period at both day 1 and week 6, improvements from baseline in FEV1 (compared with placebo) were greater with aclidinium than with tiotropium.
Patient symptoms also improved following treatment. After 6 weeks, the improvement in E-RS score was greater with aclidinium compared with both placebo and tiotropium, as indicated by changes in RS-Breathlessness, RS-Cough and Sputum, and E-RS Total scores. In addition, the percentage of patients defined as E-RS responders increased with aclidinium compared with tiotropium or placebo. Improvement over placebo and tiotropium was also observed in early-morning symptom severity, nighttime symptom severity, individual early-morning symptoms (shortness of breath and cough), and limitation of early-morning activity caused by symptoms. Safety and tolerability in symptomatic patients appeared to be similar to that in the overall study population, with the most commonly reported TEAEs being headache and nasopharyngitis.
The overall study population included both symptomatic and asymptomatic patients. Results have been previously reported and indicated that aclidinium provided significant 24-hour bronchodilation versus placebo from day 1, with comparable efficacy to tiotropium after 6 weeks.6 In this post hoc analysis, some notable differences were observed in the symptomatic patient group. Improvements in bronchodilation during the nighttime period were greater with aclidinium than with tiotropium in symptomatic patients, whereas in the overall population, no differences were observed between the two treatments. Furthermore, symptomatic patients experienced a greater reduction in nighttime symptom severity from baseline to 6 weeks with aclidinium, compared with tiotropium. In the overall population, although nighttime symptom severity was significantly reduced with aclidinium versus placebo, the difference between the two comparators was not statistically significant.6 For both the overall population and the symptomatic patient group, no differences in nighttime symptom severity were observed for tiotropium versus placebo.
While noting that the BID dosing regimen of aclidinium may have contributed to the observed improvement in nighttime efficacy versus tiotropium QD in symptomatic patients, improvement of nighttime lung function is of particular importance for patients with COPD as nighttime symptoms and poor quality sleep are common.1 To date, relatively few studies have demonstrated significant improvements in nighttime lung function and/or sleep quality following bronchodilator therapy.1,18–22
The improvements in E-RS score observed in symptomatic patients receiving treatment with aclidinium are likely to be clinically significant since E-RS has been shown to be a valid and reliable tool for the assessment of respiratory symptoms of COPD in clinical trials.11,14,23 The minimal clinically important differences for the different aspects of the E-RS tool have recently been proposed: RS-Total ≥−2 units; RS-Breathlessness ≥−1 unit; RS-Cough and Sputum ≥−0.7 units; and RS-Chest Symptoms ≥−0.7 units,14 and the ability of the E-RS to capture treatment effects has recently been evaluated.11 In this study, change in E-RS Total score was −2.8 units with aclidinium versus −0.7 units with placebo and −1.6 units with tiotropium. With aclidinium, changes from baseline in individual domain scores at week 6 were −1.3 for Breathlessness, −0.6 for Chest Symptoms, and −0.8 for Cough and Sputum.
There are some potential limitations of this post hoc analysis. There was found to be a significantly higher proportion of patients with baseline bronchial reversibility in the aclidinium group compared with the tiotropium group, which may potentially account for the significant difference in efficacy observed. The apparent higher proportion of patients with severe COPD in the tiotropium group is, however, unlikely to have influenced the FEV1 response in these patients, since the differences between groups were not found to be significant. Also, the 6-week study period may not be long enough to reflect a patient’s symptom burden. In addition, the E-RS threshold used to distinguish symptomatic from asymptomatic patients has not been formally validated and requires further investigation.11 One must also consider that unvalidated, early versions of the early-morning (Early Morning Symptoms of COPD Instrument) and nighttime symptoms (Nighttime Symptoms of COPD Instrument) questionnaires were used in this study. Both questionnaires have subsequently been developed and evaluated further, and there are published data indicating that these are valid tools for measuring COPD symptoms in large randomized trials.15,16 The use of a patient questionnaire, rather than clinical assessments, to evaluate limitation of early-morning activity could be considered a potential constraint of this study; however, the benefit of this method is that it can assess if patients are restricted in their usual morning activities, such as getting washed and dressed.
One further consideration of this post hoc analysis is that although symptomatic patients constituted 45% of the overall study population, the total sample size remains relatively small (n=414). Furthermore, it should be noted that it is possible that a different group of symptomatic patients may have been identified if the COPD Assessment Test or modified Medical Research Council criteria outlined in the GOLD report were applied.5
Conclusion
Results from this post hoc analysis of a symptomatic patient group with moderate to severe COPD showed that aclidinium 400 μg BID provided additional improvements compared with tiotropium 18 μg QD in: 1) bronchodilation, particularly during the nighttime, 2) E-RS responder status, 3) early-morning, daytime, and nighttime symptoms, and 4) early-morning limitation of activity. These results suggest that symptomatic patients may achieve greater benefits during the nighttime with aclidinium treatment than patients with fewer symptoms.
Acknowledgments
The authors would like to thank all of the patients and their families, the team of investigators, research nurses, and operations staff involved in these studies. This study was funded by Almirall S.A., Barcelona, Spain. The authors would also like to thank Jennifer Higginson of Complete Medical Communications, who provided medical writing support under the direction of the authors, funded by AstraZeneca.
Data included in this paper were presented at the British Thoracic Society Winter Meeting 2015 as a poster discussion session (abstract published in “Poster Abstracts”, Thorax 2015;70:A139 [http://thorax.bmj.com/content/70/Suppl_3/A139; DOI: 10.1136/thoraxjnl-2015-207770.263]), and at the American Thoracic Society International Conference 2016 as a poster presentation (abstract published in “American Thoracic Society International Conference Abstracts”, Am J Respir Crit Care Med 2016;193:A6817 [http://www.atsjournals.org/doi/pdf/10.1164/ajrccm-conference.2016.193.1_MeetingAbstracts.A6817; DOI: 10.1164/ajrccm-conference.2016.193.1_Meeting Abstracts.A6817]).
Footnotes
Disclosure
JB has received consulting fees, speaker’s fees, and travel expenses from AstraZeneca and has also received compensation for organizing or participating in advisory boards for Cytos, Boehringer Ingelheim, Almirall, AstraZeneca, Novartis, and Revotar Biopharmaceuticals. The institution where JB is currently employed has received compensation for the design, performance, or participation in single or multicenter clinical trials in the past 5 years from several companies including Almirall, Altana, AstraZeneca, Boehringer Ingelheim, Cytos, GSK, Meda Pharmaceuticals, Merck Sharp & Dohme, Mundipharma, Novartis, Pfizer, and Revotar Biopharmaceuticals. RM has received consulting fees, speaker’s fees, and travel expenses from Boehringer Ingelheim and has also received compensation for participating in advisory boards for Boehringer Ingelheim, Almirall, AstraZeneca, and Novartis. Furthermore, RM has received compensation for participation in multicenter clinical trials in the past 5 years from several companies including Almirall, AstraZeneca, Boehringer Ingelheim, GSK, Merck Sharp & Dohme, Mundipharma, Novartis, Pearl, Roche, and Takeda. A-MK is a current employee of Pulmonary Research Institute at LungenClinic Grosshansdorf; the institution received compensation for the design of and/or participation in clinical trials from Almirall, AstraZeneca, Boehringer Ingelheim, GSK, Novartis, Pfizer, Infinity Pharmaceuticals, TEVA, Sterna Biologicals, Chiesi, Bayer, and Takeda. Furthermore, A-MK has received consulting fees, and speaker’s fees from AstraZeneca, Boehringer Ingelheim, and Roche. FC and EGG are employees of AstraZeneca PLC, Barcelona, Spain, and former employees of Almirall S.A., Barcelona, Spain.
References
- 1.Agusti A, Hedner J, Marin JM, Barbé F, Cazzola M, Rennard S. Nighttime symptoms: a forgotten dimension of COPD. Eur Respir Rev. 2011;20(121):183–194. doi: 10.1183/09059180.00004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roche N, Chavannes NH, Miravitlles M. COPD symptoms in the morning: impact, evaluation and management. Respir Res. 2013;14:112. doi: 10.1186/1465-9921-14-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miravitlles M, Worth H, Soler Cataluna JJ, et al. Observational study to characterise 24-hour COPD symptoms and their relationship with patient-reported outcomes: results from the ASSESS study. Respir Res. 2014;15:122. doi: 10.1186/s12931-014-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehteshami-Afshar S, FitzGerald JM, Doyle-Waters MM, Sadatsafavi M. The global economic burden of asthma and chronic obstructive pulmonary disease. Int J Tuberc Lung Dis. 2016;20:11–23. doi: 10.5588/ijtld.15.0472. [DOI] [PubMed] [Google Scholar]
- 5.Global Initiative for Chronic Obstructive Lung Disease Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [updated 2017] [Accessed November 17, 2016]. Available from: http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/
- 6.Beier J, Kirsten AM, Mróz R, et al. Efficacy and safety of aclidinium bromide compared with placebo and tiotropium in patients with moderate-to-severe chronic obstructive pulmonary disease: results from a 6-week, randomized, controlled Phase IIIb study. COPD. 2013;10(4):511–522. doi: 10.3109/15412555.2013.814626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuhr R, Magnussen H, Sarem K, et al. Efficacy of aclidinium bromide 400 μg twice daily compared with placebo and tiotropium in patients with moderate to severe COPD. Chest. 2012;141(3):745–752. doi: 10.1378/chest.11-0406. [DOI] [PubMed] [Google Scholar]
- 8.Marth K, Schuller E, Pohl W. Improvements in patient-reported outcomes: a prospective, non-interventional study with aclidinium bromide for treatment of COPD. Respir Med. 2015;109(5):616–624. doi: 10.1016/j.rmed.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Beier J, Kirsten A-M, Mroz R, et al. Efficacy of aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD: a phase IIIb study. Thorax. 2012;67:A26–A27. doi: 10.3109/15412555.2013.814626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhr R, Magnussen H, Ribera Llovera A, et al. Efficacy and safety of twice-daily aclidinium bromide compared with tiotropium and placebo in patients with moderate to severe COPD. Eur Respir J. 2010;36(Suppl 54):219s. [Google Scholar]
- 11.Jones PW, Leidy NK, Hareendran A, Lamarca R, Chuecos F, Garcia Gil E. The effect of aclidinium bromide on daily respiratory symptoms of COPD, measured using the Evaluating Respiratory Symptoms in COPD (E-RS: COPD) diary: pooled analysis of two 6-month Phase III studies. Respir Res. 2016;17(1):61. doi: 10.1186/s12931-016-0372-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leidy NK, Murray LT, Jones P, Sethi S. Performance of the EXAcerbations of chronic pulmonary disease tool patient-reported outcome measure in three clinical trials of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:316–325. doi: 10.1513/AnnalsATS.201309-305OC. [DOI] [PubMed] [Google Scholar]
- 13.Leidy NK, Sexton CC, Jones PW, et al. Measuring respiratory symptoms in clinical trials of COPD: reliability and validity of a daily diary. Thorax. 2014;69(5):443–449. doi: 10.1136/thoraxjnl-2013-204428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leidy NK, Murray LT, Monz BU, et al. Measuring respiratory symptoms of COPD: performance of the EXACT-Respiratory Symptoms Tool (E-RS) in three clinical trials. Respir Res. 2014;15(1):124. doi: 10.1186/s12931-014-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mocarski M, Zaiser E, Trundell D, Make BJ, Hareendran A. Evaluation of the psychometric properties of the nighttime symptoms of COPD instrument. Int J Chron Obstruct Pulmon Dis. 2015;10:475–487. doi: 10.2147/COPD.S75776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hareendran A, Zaiser E, Make B, Garcia-Gil E. The development and psychometric validation of the early morning symptoms of COPD instrument (EMSCI) Thorax. 2016;71(Suppl 3):A204. doi: 10.2147/COPD.S152087. P218 (abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 18.Calverley PM, Lee A, Towse L, van Noord J, Witek TJ, Kelsen S. Effect of tiotropium bromide on circadian variation in airflow limitation in chronic obstructive pulmonary disease. Thorax. 2003;58(10):855–860. doi: 10.1136/thorax.58.10.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin RJ, Bartelson BL, Smith P, et al. Effect of ipratropium bromide treatment on oxygen saturation and sleep quality in COPD. Chest. 1999;115(5):1338–1345. doi: 10.1378/chest.115.5.1338. [DOI] [PubMed] [Google Scholar]
- 20.McNicholas WT, Calverley PM, Lee A, Edwards JC. Long-acting inhaled anticholinergic therapy improves sleeping oxygen saturation in COPD. Eur Respir J. 2004;23(6):825–831. doi: 10.1183/09031936.04.00085804. [DOI] [PubMed] [Google Scholar]
- 21.Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1993;148(4 Pt 1):1030–1036. doi: 10.1164/ajrccm/148.4_Pt_1.1030. [DOI] [PubMed] [Google Scholar]
- 22.Ryan S, Doherty LS, Rock C, Nolan GM, McNicholas WT. Effects of salmeterol on sleeping oxygen saturation in chronic obstructive pulmonary disease. Respiration. 2010;79(6):475–481. doi: 10.1159/000235619. [DOI] [PubMed] [Google Scholar]
- 23.Leidy NK, Kimel M, Ajagbe L, Kim K, Hamilton A, Becker K. Designing trials of behavioral interventions to increase physical activity in patients with COPD: insights from the chronic disease literature. Respir Med. 2014;108(3):472–481. doi: 10.1016/j.rmed.2013.11.011. [DOI] [PubMed] [Google Scholar]