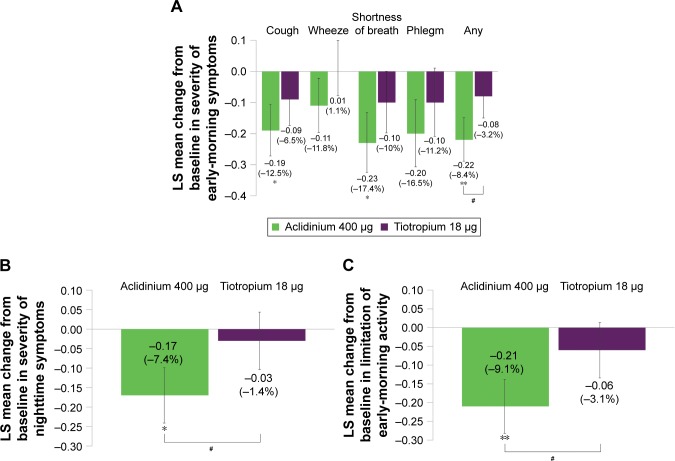
Figure 5.
Symptomatic patients: change from baseline in symptom severity in the early morning (A) and nighttime (B), and limitation of early-morning activity (C) over 6 weeks.
Notes: Percentage reduction is shown in brackets. Placebo N=60, aclidinium 400 μg N=116, tiotropium 18 μg N=101. *P<0.05, **P<0.01 versus placebo; #P<0.05 versus tiotropium. Specific symptoms (A): 1= mild, 2= moderate, 3= severe, 4= very severe; any symptom: 1= no symptoms, 2= mild, 3= moderate, 4= severe, 5= very severe. For patients with no symptom, a zero value was assigned. Nighttime symptom severity (B): 1= mild, 2= moderate, 3= severe, 4= very severe. Limitation of activity (C): 1= not at all, 2= a little, 3= moderately, 4= a good deal, 5= a very good deal.
Abbreviation: LS, least squares.