Figure 4.
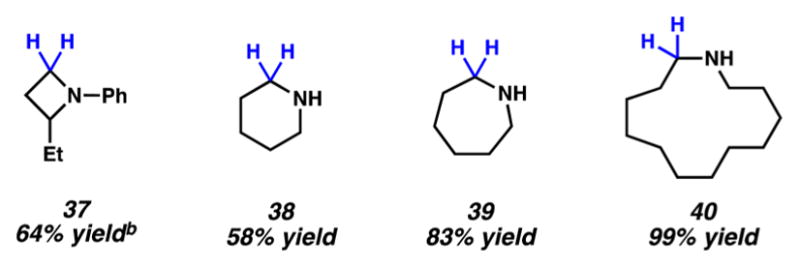
Cyclic amines prepared by reduction of the corresponding lactams. (a) Conditions unless otherwise stated: NiCl2(dme) (10 mol %), substrate (1.0 equiv, 0.2 mmol), PhSiH3 (2.0 equiv), and toluene (1.0 M) at 115 °C for 24 h in a sealed vial. Yield determined by 1H NMR analysis using hexamethylbenzene as an internal standard due to product volatility. (b) Yield shown reflects the average of two isolation experiments.
