Abstract
Background:
CD4+ T-cells play important roles in the pathogenesis of Hashimoto's thyroiditis (HT). However, there is limited data about characteristics and function of the newly interleukin (IL)-17–producing T-helper cells in this common autoimmune thyroid disorder.
Aim:
The purpose of this study was to assess the levels of T-helper 17-related cytokines in sera of patients with HT.
Materials and Methods:
Cytokine concentrations were measured in 48 patients with overt (n = 23) and subclinical hypothyroidism (25) and 35 healthy controls using enzyme-linked immunosorbent assay.
Results:
The serum levels of IL-17 were significantly higher in patients than controls (P = 0.001) while no differences were observed with regard to levels of IL-22 and IL-23 between patients and normal controls.
Conclusion:
These results suggest that IL-17 could play some role in the pathogenesis of HT.
Keywords: Autoimmunity, cytokine, Hashimoto's thyroiditis, T-lymphocyte
INTRODUCTION
Hashimoto's thyroiditis (HT) is one of the most prevalent autoimmune endocrine disorders, characterized by lymphocytic infiltration and fibrosis of the thyroid gland. The etiology of disease is unclear, however, it is thought that both genetic and environmental risk factors influence on the development of inappropriate immune responses against thyroid.[1] Evidence shows an association between the magnitude of cell-mediated immunity and the increased likelihood of HT development.[2] It is also noteworthy that thyroid cells are attacked by distinct subsets of effector CD4+ T-helper (Th) cells with diverse effector functions. However, the exact role of these different Th cell subpopulations is still not well understood.
Classically, HT is considered a Th1-mediated disease, similar to autoimmune diseases such as multiple sclerosis and vitiligo.[3,4,5] This classification has changed overtime due to the identification of new Th cell subsets including Th17 cells. According to the new study findings, uncontrolled Th17 cell responses have been implicated in different types of autoimmune diseases, which were formerly assumed to be Th1-dependent diseases.[6] Nonetheless, these cells are not per se harmful. In fact, they act as a double-edged sword and mediate both normal and pathological immune responses. On the one hand, they play a major role in host defense against extracellular microbes. On the other hand, they are involved in the pathogenesis of several autoimmune diseases.[7] Th17 cells exhibit cell type-specific patterns of cytokine secretion. They are a major source of interleukin (IL)-17 and IL-22 and their effector functions are mediated mainly by these secreted cytokines. Moreover, IL-23 is primarily required for expansion and survival of the Th17 cell population.[8] Thus, the objective of this project was (1) to identify whether there was a difference in the circulating levels of Th17-related cytokines (IL-17, IL-22, and IL-23) between patients with HT and healthy controls (HCs) (2) to examine the relationship or association between variables measured in this study.
MATERIALS AND METHODS
Sampling
Blood samples were collected from 48 drug-naive and newly diagnosed patients suffering from HT and 38 normal controls. Serum specimens were separated by centrifugation and then stored at −20°C until assay. According to thyroid status, individuals grouped as overt hypothyroidism (OHT), subclinical hypothyroidism (SCHT), and HC. OHT was defined as an elevated thyroid-stimulating hormone (TSH) >10 mIU/L in conjunction with a positive anti-thyroid peroxidase antibodies (TPO) antibodies (>40 IU/mL), SCHT was characterized by a TSH between 4 and 10 mIU/L in combination with anti-TPO antibodies (>40 IU/ml). Thyroid function was declared normal when TSH fell within the normal reference ranges.
Inclusion criteria were age between 18 and 75 years, clinical and biochemical confirmation of hypothyroidism as HT and signed written informed consent form before participating in the experiment.
Criteria for exclusion included (1) pregnant women or breastfeeding mother, (2) known cases of preexisting thyroid dysfunction or surgery, (3) presence of malignancy, chronic inflammatory, and autoimmune diseases except HT (4) the coexistence of acute or chronic infection with or without fever (5) history of immunosuppressive therapy or drug use that might influence thyroid function such as lithium or iodide and finally users of cigarettes, cigars, or smokeless tobacco.
The mean age of the patients (male 13, female 35) was 42.12 ± 12 years. A group of age- and sex-matched healthy controls with no history of underlying malignancy, autoimmune, or inflammatory diseases were included in the study. They consisted of ten men and 25 women with a mean age of 43.7 ± 14.27 years. The study protocol was fully approved by the Ethics Committee of Tehran University of Medical Sciences through reference no. IR.TUMS.REC.1395.00108. All participants gave informed written consent before enrollment to the study.
Thyroid function assessment
In this study, free thyroxine (FT4), TSH, and anti-TPO were measured in all patients and HC. Serum FT4 concentrations were determined using a competitive enzyme immunoassay kit (Monobind Inc., Lake Forest, USA). The intra- and inter-assay coefficient of variation were 10.98% and 10.81%, respectively. The sensitivity of the test was 0.3 ng/dl. Normal reference ranges for FT4 were 0.8–2.0 ng/dl. Serum TSH (normal value: 0.2–4 μIU/ml) was examined by immunoradiometric assay (TSH IRMA, Radim, Pomezia, Rome, Italy). The assay of antithyroid peroxidase (anti-TPO) antibodies was also determined, using the diagnostic enzyme-linked immunosorbent assay (ELISA) kit (Monobind Inc., Lake Forest, USA). Values in excess of 40 IU/ml were considered positive for the presence of anti-TPO.
Cytokine assay
IL-17A, IL-22, and IL-23 serum measurements were detected using an ELISA method according to the manufacturer's instructions (Diaclone, Besançon, France).
Statistical analysis
Independent sample t-test was used for comparing the means of the two groups. Correlation analysis was performed using Pearson's test. P < 0.05 was considered statistically significant. Results are expressed as means ± standard deviation.
RESULTS
Patients and control groups were similar to each other in terms of age (P = 0.59) and sex (P = 0.88) distribution. In addition, the disease was 2.7-fold higher in women than in men. Patients had a significantly higher TSH (P = 0.001) concentrations than controls (P < 0.001) while FT4 levels did not differ among the groups (P = 0.68) [Table 1]. Only overt hypothyroid patients showed significantly decreased serum FT4 levels as compared to the patients with SCHT (P = 0.04).
Table 1.
Comparisons of laboratory findings and demographic characteristics of total Hashimoto's thyroiditis, subclinical hypothyroid, overt hypothyroid, and healthy control groups
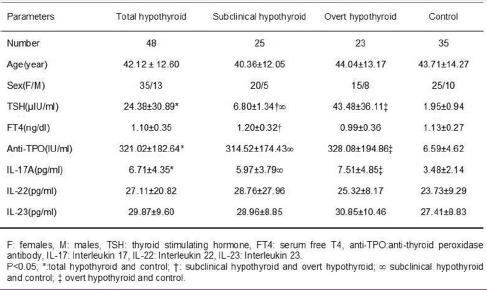
Pearson's correlation analysis revealed a significant negative correlation between circulating TSH and FT4 concentrations in total HT group (r = −0.450, P = 0.001). Subgroup analysis for the correlation between TSH and FT4 indicated a negative correlation only in patients with OHT (r = −0.467, P = 0.025) but not in patients with SCHT (r = −0.273, P = 0.186).
Both subclinical and overt hypothyroid patients had higher anti-TPO antibody titers than controls (P = 0.001). However, the level of anti-TPO antibody did not differ between subclinical hypothyroid and overt subgroups.
Serum levels of T-helper 17-related cytokines
As shown in Figure 1, all patients with HT exhibited higher levels of serum IL-17A compared to those of HCs. Levels of IL-17A were also significantly increased in overt and subclinical forms of hypothyroidism compared to control individuals (P < 0.05). Nonetheless, there were no statistically significant differences between patients with overt and SCHT in serum levels of IL-17A. The mean serum IL-22 and IL-23 levels in the patient groups were higher than in HCs, but the differences were not statistically significant [Figure 2].
Figure 1.
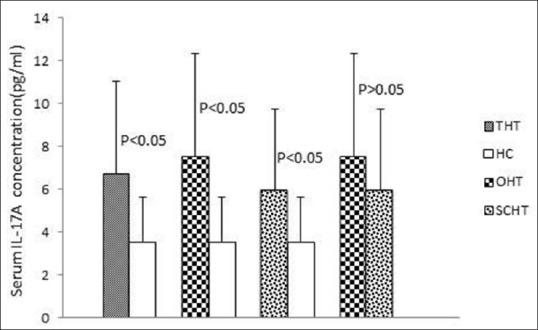
Serum interleukin-17A levels in study groups
Figure 2.
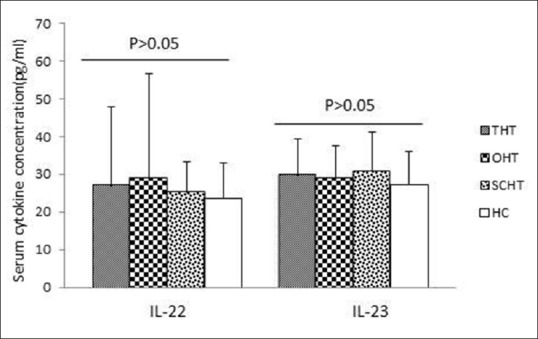
Serum interleukin-22 and interleukin-23 levels in study groups
Correlation analysis revealed no significant relationships between IL-23 and IL-22 or IL-17 levels. In addition, no significant correlation was observed between circulating IL-22 and IL-17A concentrations in total HT group and patients with SCHT. However, IL-22 was positively correlated with IL-17A in patients with OHT (r = 0.482, P = 0.020). Moreover, circulating levels of IL-17, IL-22, and IL-23 also failed to correlate with other clinical parameters such as TSH, FT4, and anti-TPO within the patient groups.
DISCUSSION
Recent developments in molecular techniques have revolutionized our understanding of the main cellular components of the immune system that determine the fate of immune response. These methods have revealed the presence of different subsets of lymphocytes each having a distinct function, specific cell surface markers, cytokine profiles, and differential developmental requirements. Th17 cells are a recently discovered unique lineage of CD4+ T-cells. The identification of these cells that have both offensive and defensive capabilities provides new insight into the pathogenesis of autoimmune diseases, especially those that are considered to be the result of aberrant Th1 responses.[9] They are the major source of IL-17A, IL-22. IL-23 seems to play a pivotal role in their development and persistence.[10] Therefore, quantification of cytokines that are essential for the development and function of Th17 cells in health and disease may be critical for understanding the complex molecular mechanisms underlying HT. The results of the present study demonstrate a significant increase in the serum IL-17 levels of HT patients, suggesting a potential role of this cytokine in disease pathogenesis. It must be mentioned that IL-17 is a proinflammatory cytokine and can also induce the expression of diverse proinflammatory cytokines and chemokines.[11] These inflammatory molecules are responsible for the formation of an inflammatory milieu which augment disease progression by different pathways such as development of fibrosis. The relationship between fibrosis of the thyroid gland and augmented inflammatory mediators is confirmed by scientists such as Li et al.[12] They indicated an association between the expression of IL-17 and stromal fibrosis in patients' thyroid glands. The importance of IL-17 in the pathogenesis of HT has further been verified by different clinical trials and also supported by experimental models of disease. For instance, thyroid accumulation of Th17 cells in the murine experimental model of human HT with IL-17+/+ T-cells but not in those with IL-17-/-T-cells indicates the role of IL-17 in HT pathophysiology.[13]
IL-22 is another proinflammatory cytokine that acts principally on nonimmune epithelial and stromal cells that express receptors for them. Like IL-17, IL-22 has the potential of inducing proinflammatory cytokines and is associated with inflammatory disorders.[14] Therefore, it may also be involved in the development of thyroid abnormalities in patients with HT. In the present study, HT patients displayed higher levels of IL-22 in comparison with normal controls, but this difference was not statistically significant. These results are in agreement with limited earlier studies that detected increased levels of IL-22 in patients with HT. For instance, Figueroa-Vega et al. indicated a significant increase in the serum levels of IL-22 and percentage of CD3+CD8−IL-22+T-cells in the peripheral blood mononuclear cells (PBMCs) of patients with HT.
Moreover, immunohistochemistry revealed a stronger expression of IL-22 in the thyroid gland of HT patients compared with Graves' disease or control subjects.[15] In another study, Ruggeri and coworkers indicated a statistically significant increase in the serum levels of IL-22 in patients with HT in comparison with normal controls and non-HT-goitrous patients.[16] Therefore, the changes of IL-22 level may be related to the pathogenesis of HT.
The final part of this study was devoted to determination of serum levels of IL-23 as a determining cytokine for Th17 cells development. Our results showed that HT patients have high levels of IL-23 in comparison with normal controls. However, there were no significant differences between the two groups. These findings are nearly similar to the previous data from the literature and emphasize the critical role of IL-23 in the pathogenesis of HT. For instance, Qin et al. indicated increased expression of IL-23 in HT thyroid tissues, which was accompanied by corresponding increase in IL-17A.[17] Similarly, elevated levels of IL-17 and IL-23 in PBMCs and serum have been demonstrated in patients with HT compared with HC.[18,19,20] These results provide evidence for the importance of the IL-17/IL-23 axis in the pathogenesis of HT. Nonetheless, some researchers believe that the activity of this axis is dependent on the stage of disease[18,19] and others suggest a mixed response from Th17 and Th1 in HT, rather than one of them.[17] Patients in the present study were all newly diagnosed regardless of disease stage. In our opinion, it is one of the several limitations in this research and several other-related projects. Therefore, further studies are needed to investigate the role of Th17 cells in HT according to the stages of disease.
Another limitation of this study was the lack of data about the production and mRNA expression of the Th17-related cytokines by PBMC. Moreover, we did not evaluate the cytokines profile (IL-23, IL-22, and IL-17A) within the thyroid gland of the patients. Therefore, more studies are required to elucidate the role of Th17-related cytokines in the thyroid of patients with HT.
It is well known that IL-23 is not the only cytokine that induces Th17 development and other inflammatory cytokines (e.g., IL-6, IL-1, and transforming growth factor-β) are known to play a role in the differentiation of these cells.[21] As a result, simultaneous assessment of these cytokines improve our understanding of the mechanisms underlying HT progression by Th17-dependent mechanisms. Finally, we should point out here that many researchers indicating that other cells of the immune system (such as Th1, Th2, Tregulatory) are also involved in HT pathogenesis. Thus, further studies are needed to elucidate the participation of different T-cell subpopulations and their cytokines in disease pathogenesis.
CONCLUSION
The present study demonstrates altered pattern of Th17-related cytokines in patients suffering from HT. As a result, further studies using large numbers of patients are required to confirm these findings.
Financial support and sponsorship
This project was conducted with the financial support of Tehran University of Medical Sciences for Research through contract no. 1942.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14:174–80. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Tokic S, Š tefanic M, Glavaš-Obrovac L, Jaman S, Novosadová E, Petrkova J, et al. The expression of T cell FOXP3 and T-bet is upregulated in severe but not euthyroid Hashimoto's thyroiditis. Mediators Inflamm. 2016;2016:3687420. doi: 10.1155/2016/3687420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanba T, Watanabe M, Inoue N, Iwatani Y. Increases of the Th1/Th2 cell ratio in severe Hashimoto's disease and in the proportion of Th17 cells in intractable Graves' disease. Thyroid. 2009;19:495–501. doi: 10.1089/thy.2008.0423. [DOI] [PubMed] [Google Scholar]
- 4.Nouri-Koupaee A, Mansouri P, Jahanbini H, Sanati MH, Jadali Z. Differential expression of mRNA for T-bet and GATA-3 transcription factors in peripheral blood mononuclear cells of patients with vitiligo. Clin Exp Dermatol. 2015;40:735–40. doi: 10.1111/ced.12661. [DOI] [PubMed] [Google Scholar]
- 5.Frisullo G, Plantone D, Marti A, Iorio R, Damato V, Nociti V, et al. Type 1 immune response in progressive multiple sclerosis. J Neuroimmunol. 2012;249:112–6. doi: 10.1016/j.jneuroim.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Bedoya SK, Lam B, Lau K, Larkin J., 3rd Th17 cells in immunity and autoimmunity. Clin Dev Immunol. 2013;2013:986789. doi: 10.1155/2013/986789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemdan NY, Abu El-Saad AM, Sack U. The role of T helper (TH) 17 cells as a double-edged sword in the interplay of infection and autoimmunity with a focus on xenobiotic-induced immunomodulation. Clin Dev Immunol. 2013;2013:374769. doi: 10.1155/2013/374769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zúñiga LA, Jain R, Haines C, Cua DJ. Th17 cell development: From the cradle to the grave. Immunol Rev. 2013;252:78–88. doi: 10.1111/imr.12036. [DOI] [PubMed] [Google Scholar]
- 9.Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res. 2016;2016:7692024. doi: 10.1155/2016/7692024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–14. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beringer A, Noack M, Miossec P. IL-17 in chronic inflammation: From discovery to targeting. Trends Mol Med. 2016;22:230–41. doi: 10.1016/j.molmed.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Li D, Cai W, Gu R, Zhang Y, Zhang H, Tang K, et al. Th17 cell plays a role in the pathogenesis of Hashimoto's thyroiditis in patients. Clin Immunol. 2013;149:411–20. doi: 10.1016/j.clim.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Horie I, Abiru N, Nagayama Y, Kuriya G, Saitoh O, Ichikawa T, et al. T helper type 17 immune response plays an indispensable role for development of iodine-induced autoimmune thyroiditis in nonobese diabetic-H2h4 mice. Endocrinology. 2009;150:5135–42. doi: 10.1210/en.2009-0434. [DOI] [PubMed] [Google Scholar]
- 14.Perusina Lanfranca M, Lin Y, Fang J, Zou W, Frankel T. Biological and pathological activities of interleukin-22. J Mol Med (Berl) 2016;94:523–34. doi: 10.1007/s00109-016-1391-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figueroa-Vega N, Alfonso-Pérez M, Benedicto I, Sánchez-Madrid F, González-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto's thyroiditis. J Clin Endocrinol Metab. 2010;95:953–62. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- 16.Ruggeri RM, Minciullo P, Saitta S, Giovinazzo S, Certo R, Campennì A, et al. Serum interleukin-22 (IL-22) is increased in the early stage of Hashimoto's thyroiditis compared to non-autoimmune thyroid disease and healthy controls. Hormones (Athens) 2014;13:338–44. doi: 10.14310/horm.2002.1483. [DOI] [PubMed] [Google Scholar]
- 17.Qin Q, Liu P, Liu L, Wang R, Yan N, Yang J, et al. The increased but non-predominant expression of Th17- and Th1-specific cytokines in Hashimoto's thyroiditis but not in Graves' disease. Braz J Med Biol Res. 2012;45:1202–8. doi: 10.1590/S0100-879X2012007500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konca Degertekin C, Aktas Yilmaz B, Balos Toruner F, Kalkanci A, Turhan Iyidir O, Fidan I, et al. Circulating Th17 cytokine levels are altered in Hashimoto's thyroiditis. Cytokine. 2016;80:13–7. doi: 10.1016/j.cyto.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Wang H, Su Z, Chen J, Xue Y, Wang S, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto's thyroiditis. Scand J Immunol. 2010;72:250–5. doi: 10.1111/j.1365-3083.2010.02425.x. [DOI] [PubMed] [Google Scholar]
- 20.Ruggeri RM, Saitta S, Cristani M, Giovinazzo S, Tigano V, Trimarchi F, et al. Serum interleukin-23 (IL-23) is increased in Hashimoto's thyroiditis. Endocr J. 2014;61:359–63. doi: 10.1507/endocrj.ej13-0484. [DOI] [PubMed] [Google Scholar]
- 21.Hemdan NY, Birkenmeier G, Wichmann G, Abu El-Saad AM, Krieger T, Conrad K, et al. Interleukin-17-producing T helper cells in autoimmunity. Autoimmun Rev. 2010;9:785–92. doi: 10.1016/j.autrev.2010.07.003. [DOI] [PubMed] [Google Scholar]


