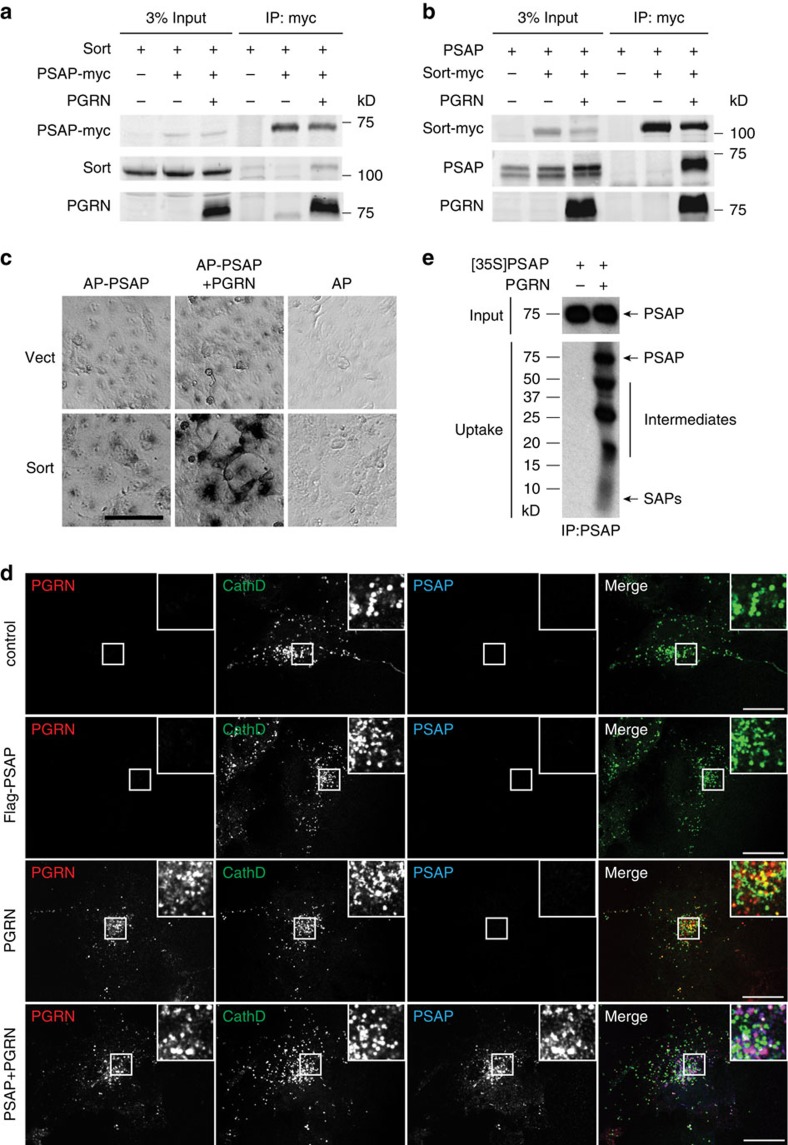
Figure 1. PGRN bridges the interaction between PSAP and sortilin and facilitates lysosomal targeting of PSAP via sortilin.
(a) Myc-tagged PSAP-, PGRN- and sortilin (Sort)-expressing constructs were transfected into HEK293T cells as indicated. Cell lysates were subject to anti-myc immunoprecipitation and blotted with anti-sortilin, myc and PGRN antibodies. (b) PSAP, PGRN and myc-tagged sortilin (Sort)-expressing constructs were transfected into HEK293T cells as indicated. Cell lysates were subject to anti-myc immunoprecipitation and blotted with anti-sortilin, myc and PGRN antibodies. (c) COS-7 cells transfected with an empty vector (Vect) or sortilin (Sort)-expressing construct were incubated with AP-tagged PSAP alone or AP-PSAP with PGRN. Scale bar, 100 μm. (d) Sortilin-expressing COS-7 cells were treated with recombinant FLAG-PSAP (1 μg ml−1) and/or his-PGRN (1 μg ml−1) as indicated at 37 °C for 5 h. Cells were costained with anti-FLAG, anti-PGRN and anti-cathepsin D antibodies. Scale bar, 20 μm. (e) Sortilin-expressing COS-7 cells were incubated with radiolabelled CM containing PSAP with or without recombinant his-PGRN (1 μg ml−1) for 24 h before lysis and immunoprecipitation with anti-PSAP antibodies. The immunoprecipitation products were separated on tricine gels and visualized by radiography. (a–e) The representative images from three independent experiments.