Figure 3.
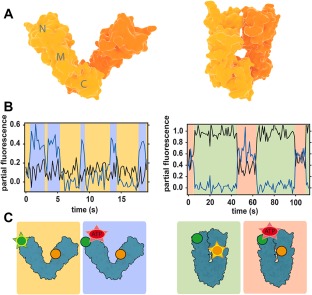
Conformational changes of Hsp90 studied with FRET. (A) Crystal structures of open (left) and closed (right) conformations of bacterial Hsp90 dimer (monomers are indicated by different color shades). (B) Partial fluorescence traces of two acceptors in 3‐colour FRET experiments: black line corresponds to the NTD acceptor, blue line to nucleotide acceptor. The traces are calculated by dividing acceptor intensity by the total fluorescence signal.46 Data shows that nucleotides can bind Hsp90 dimer in both open and closed conformations. (C) Scheme of the conformations and labeling of Hsp90. Green circle is donor, yellow is acceptor monitoring NTD dynamics and red is the nucleotide acceptor. Emission is represented by a star. Background colors link each conformation to the corresponding portion of the fluorescence traces in (B). Figures (B) and (C) are redrawn from Ref. 46.
