Figure 1.
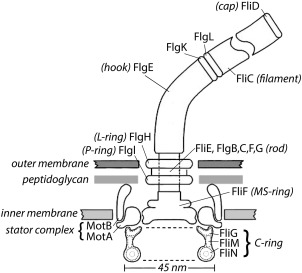
A scale drawing of the base of the E. coli flagellum, embedded in three layers of the cell wall. The outer and inner layers are fluid, but the intermediate layer, the peptidoglycan, is rigid, which gives the cell the shape of a rod with semi‐spherical end caps. The external components of the flagellum include the filament with distal cap and the hook, and the internal components include the rod, the L‐ and P‐rings, the MS‐ring, the C‐ring, and the stator complexes. The filament (a polymer of the protein FliC, also called flagellin) is shown broken, since it is several μm long. Polymerization occurs under the distal cap (FliD). Two adapter proteins (hook‐associated proteins, FlgK and FlgL) enable the hook (FlgE) to flex and the filament to rotate rigidly. The filament is a propeller that exhibits different polymorphic forms depending upon direction of rotation and torsional load, while the hook is a flexible coupling (or universal joint). A flexible coupling is required because the hooks project from the sides of the cell while the bundle of filaments (∼4 in number) that pushes the cell forward tends to align with the long axis of the cell. The rod (or drive shaft: FliE, FlgB,C,F, and G) is connected to the hook at its distal end and to the MS‐ring at its proximal end. The rod passes through the L‐ and P‐rings (FlgH and FlgI), which are mounted in the lipopolysaccharide and peptidoglycan layers, respectively, and are thought to serve as bushings. Torque is generated when protons flow from the outside to the inside of the cell through two channels in a stator complex, bounded by 4 MotA and 2 MotB. Each stator complex – there are as few as 1 or as many as 11 – is linked to the peptidoglycan by the C‐terminus of MotB and interacts electrostatically via a cytoplasmic domain of MotA with a ridge in FliG farthest from the axis of rotation. Other components of the C‐ring, FliM and FliN, interact with the signaling molecule of the chemotaxis network, CheY‐P, to control the direction of rotation. At room temperature, the direction of rotation in the absence of CheY‐P is counterclockwise (the direction of rotation of the rod when viewed from outside of the cell, i.e., from the top in this figure). Each motor comprises 26 copies of FliF and FliG, 34‐45 copies of FliM, and 34‐45 tetramers of FliN. The FliN tetramers appear as donuts in the cross‐sectional view of the C‐ring shown here. Not shown: CheY‐P; FliH, an export component known to interact with FliN; FliL, a component that enhances torque and interacts with the stator complex and with the MS‐ring; and the flagellar export apparatus that coordinates the export of axial flagellar components and is mounted at the center of the cytoplasmic face of the MS‐ring. This figure is Figure 1 from Ref. 19.
