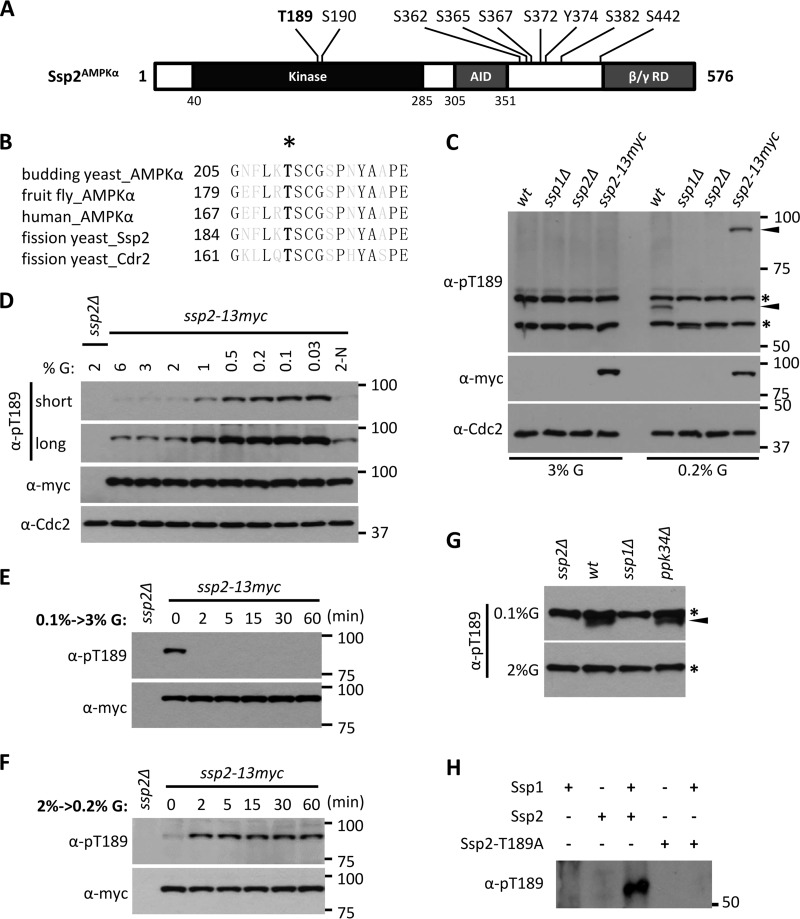
FIG 1.
Ssp1 phosphorylates Ssp2-T189 according to environmental glucose. (A) Ssp2/AMPKα phosphorylation sites identified in our recent phosphoproteomics study (29). T189 and S190 reside in the activation loop (T loop). AID, autoinhibitory domain; β/γ RD, regulatory domain of AMPK β and γ subunits. (B) Sequence alignment of activation loops of AMPKα from different species, as well as Cdr2. The black letters represent invariant residues; the asterisk denotes phosphorylated threonine. (C) Glucose-dependent detection of Ssp2-pT189. The indicated strains (wt, wild type) were grown in high (3%) or low (0.2%) glucose, and whole-cell extracts were blotted with the indicated antibodies. Anti-Cdc2 was used as a loading control. For anti-Ssp2-pT189, the asterisks denote background bands, and the arrowheads mark Ssp2-pT189 bands. (D) Ssp2-pT189 monitors glucose concentrations. Cells were shifted from 2% glucose (G) to the indicated glucose concentrations for 6 h, and then whole-cell extracts were blotted. 2-N, medium containing 2% glucose but lacking a nitrogen source. Both short and long exposures are shown for Ssp2-pT189. Anti-Cdc2 was used as a loading control. (E) Rapid dephosphorylation of Ssp2-pT189 upon shift from low (0.1%) to high (3%) glucose. The samples were whole-cell extracts. (F) Rapid phosphorylation of Ssp2-T189 upon shift from high (2%) to low (0.2%) glucose. The samples were whole-cell extracts. (G) Detection of Ssp2-pT189 in whole-cell extracts from the indicated strains grown under low (0.1%) or high (2%) glucose. The asterisks indicate background bands, and the arrowhead marks a specific Ssp2-pT189 band. (H) In vitro kinase assay using the indicated recombinant full-length proteins, which were all expressed and purified from bacteria.