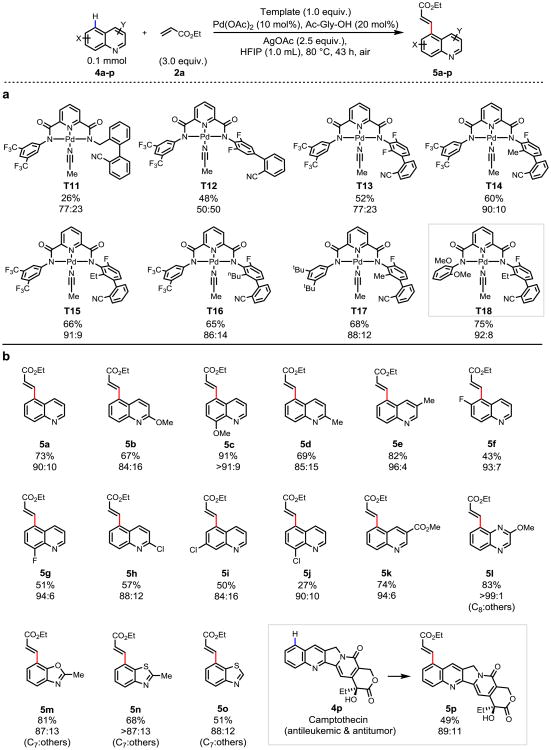
Figure 4. Remote site-selective C–H olefination of heterocycles using a non-covalent template.
a, Template evaluation. b, Substrate scope. The percentages under each structure indicate the yields of the isolated olefinated products (unless otherwise noted). The ratio of the major product to other isomers (C5:others, unless otherwise noted) were determined by 1H NMR analysis of the unpurified reaction mixture (assisted with GC-MS analysis), the variance is estimated to be within 5%. For template evaluation (a): The yields were determined by 1H NMR analysis of the unpurified reaction mixture using 1,3,5-trimethoxybenzene as the internal standard. Reaction conditions for template evaluation (a): 4a (0.1 mmol), template (1.0 equiv.), Pd(OAc)2 (15 mol%), Ac-Gly-OH (20 mol%), AgOAc (2.0 equiv.), 2a (3.0 equiv.), HFIP (1.0 mL), 100 °C, 12 h. Reaction conditions for substrate scope (b), (unless otherwise noted): substrates (0.1 mmol), T18 (1.0 equiv.), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), AgOAc (2.5 equiv.), 2a (3.0 equiv.), HFIP (1.0 mL), 80 °C, 43 h. For 5b, 5d, 5h and 5p: T14 was used as template. For 5k and 5l: 22 h. For 5m: 20 h. For 5p: 48 h, AgOAc (5.0 equiv.).