TABLE 1. Measurement and Subject Characteristics.
| Training Data | Testing Data | ||||
|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 | Cohort 3 | |
| Measurement Characteristics | |||||
| Device | Omron | Microlife | Omron | Microlife | Microlife |
| Reference | Invasive | Invasive | Invasive | Invasive | Auscultation |
| # of Subjects | 20 | 37 | 58 | 11 | 19 |
| # of Baseline Measurements | 20 | 37 | 36 | 11 | 19 |
| # of Nitroglycerin Measurements | 8 | 36 | 32 | 11 | 0 |
| # of Repeated Measurements | 0 | 73 | 0 | 16 | 16 |
| Total # of Measurements | 28 | 146 | 68 | 38 | 35 |
| Subject Characteristics | |||||
| Type | Cardiac Catheterization | Normal | |||
| Age [years] | 65.1±15.4 | 65.2±12.3 | 59.8±15.0 | 69.0±12.4 | 34.0±9.4 |
| Weight [kg] | 64.5±12.4 | 74.6±13.4 | 70.12±11.5 | 68.9±14.2 | 60.4±15.8 |
| Height [cm] | 164.0±6.4 | 163.6±8.0 | 161.6±7.8 | 162.2±10.4 | 164.2±9.3 |
| Waist circumference [cm] | 84.6±11.4 | 90.0±12.3 | 91.8±9.5 | 97.2±11.9 | 75.8±11.2 |
| Men [%] | 85 | 75.7 | 74.1 | 72.7 | 36.8 |
| Smoking [%] | 35 | 18.9 | 20.7 | 27.3 | N/A |
| Clinical diagnosis [%] | |||||
| Hypertension | 65 | 59.5 | 56.9 | 90.9 | N/A |
| Type 2 Diabetes Mellitus | 20 | 29.7 | 31 | 54.5 | N/A |
| Dyslipidemia | 45 | 37.8 | 41.4 | 36.4 | N/A |
| Coronary Artery Disease | 40 | 59.5 | 56.9 | 63.6 | N/A |
| Chronic Renal Failure | 5 | 2.7 | 3.4 | 18.2 | N/A |
| Medications [%] | |||||
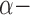 Blockers Blockers |
20 | 13.5 | 12.1 | 27.3 | N/A |
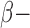 Blockers Blockers |
30 | 43.2 | 37.9 | 63.6 | N/A |
| Calcium Channel Blockers | 25 | 48.6 | 41.4 | 27.3 | N/A |
| Diuretics | 20 | 18.9 | 20.7 | 36.4 | N/A |
| Antiplatelet Agents | 65 | 86.5 | 70.7 | 81.8 | N/A |
