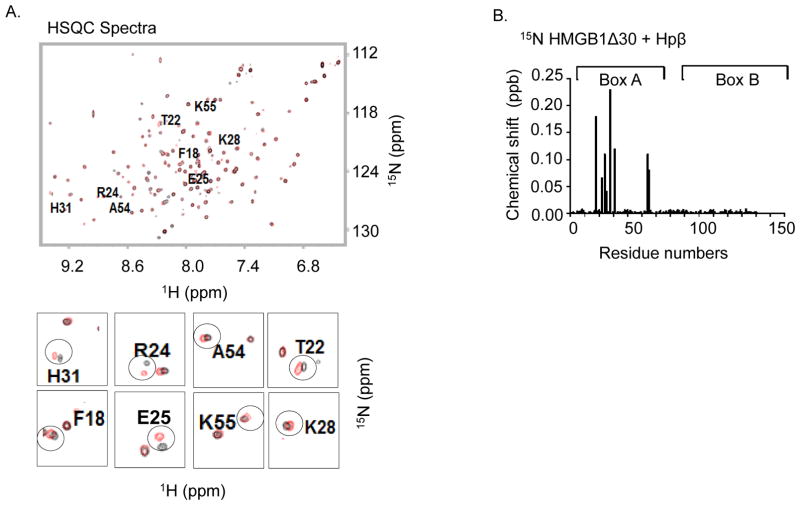
Figure 2. Nuclear magnetic resonance (NMR) analysis of disulfide Δ30-HMGB1 binding with human haptoglobin β.
(A). Upper: 15N-1H- Heteronuclear Single Quantum Coherence (HSQC) spectra for Δ30-HMGB1 in the presence or absence of haptoglobin β. HMGB1 residues (8 amino acids) with significant chemical shift perturbations due to haptoglobin β binding are labeled. Lower: enlarged 15N-HSQC spectra of Δ30-HMGB1 free (black) and in complex with haptoglobin β (red). The 8 residues showing chemical shift changes seen with disulfide Δ30-HMGB1 upon complex formation with haptoglobin β are circled. Non-overlapping black and red resonance spectra indicate a significant chemical shift; hence, an interaction between proteins via that amino acid.
(B) Chemical shift perturbation of disulfide Δ30-HMGB1 in complex with haptoglobin β is plotted as a function of residue number of HMGB1. The majority of interacting amino acids are located within HMGB1 Box A. Data are representative from 4 independent analyses.