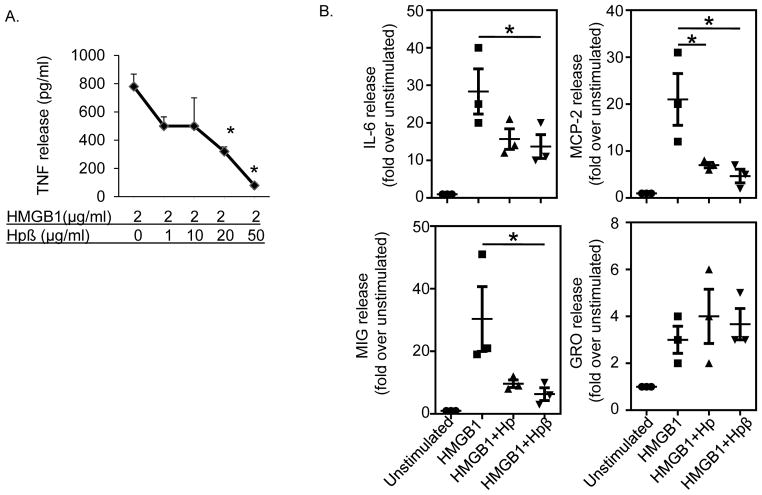
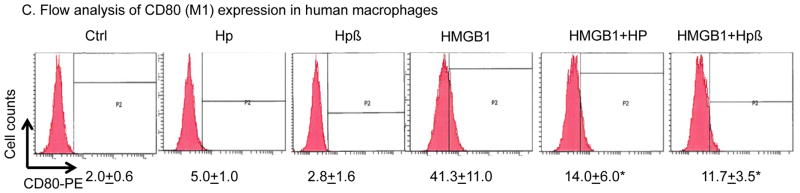
Figure 3. Sequestration of HMGB1 by haptoglobin β subunit in vitro.
(A) RAW 264.7 cells in 96-well culture plates were stimulated with HMGB1 in combination with various amounts of recombinant haptoglobin β for 16 hours. TNF released in the supernatants were measured. The effective concentration of haptoglobin β that inhibited 50% of released TNF (EC50) was approximately at 10 μg/ml (n = 5 experiments). *: p<0.05 vs. HMGB1 alone.
(B) Primary human macrophages in culture plates were stimulated with HMGB1 (1 μg/ml) plus haptoglobin or haptoglobin β at 50 μg/ml for 16 hours. Cytokine released in the supernatants were analyzed by using human cytokine antibody array. The addition of haptoglobin or haptoglobin β inhibited HMGB1-induced IL-6, MIG, MCP-2 (but not GRO) release from macrophages. Data shown are from three separate experiments. *: p<0.05 vs. HMGB1 alone.
(C) Haptoglobin (and β subunit) inhibits HMGB1-induced M1 marker expression. Human primary macrophages (106 cells per assay) were suspended in 100 μl PBS and incubated with HMGB1 (1 μg/ml) alone or plus haptoglobin (3 μg/ml) or haptoglobin β (1 μg/ml) for 30 minutes at 37°C. Cells were incubated for 10 min at 4°C with PBS containing Fc blocker rat anti-mouse CD16/CD32 (25 μg/ml) and subsequently stained with anti-CD80-PE (10 μg/ml) for 45 minutes in the dark at 4°C. Cells were washed twice with PBS and immediately analyzed on a FACS Calibur flow cytometer (n = 5 repeats). *: P<0.05 vs. HMGB1 alone.