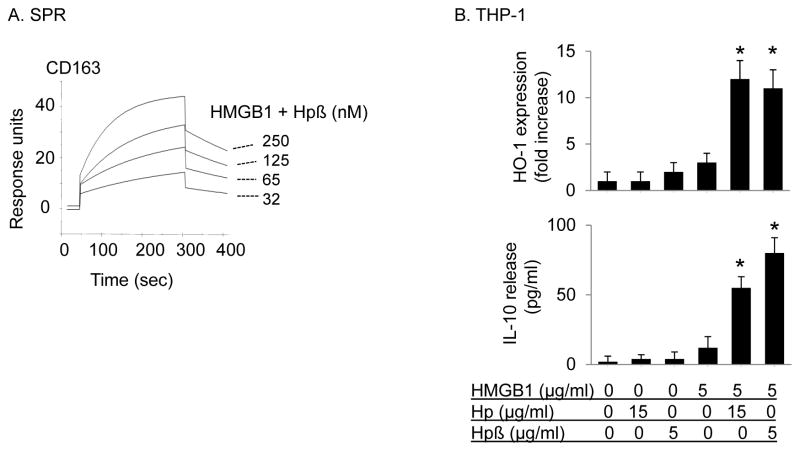
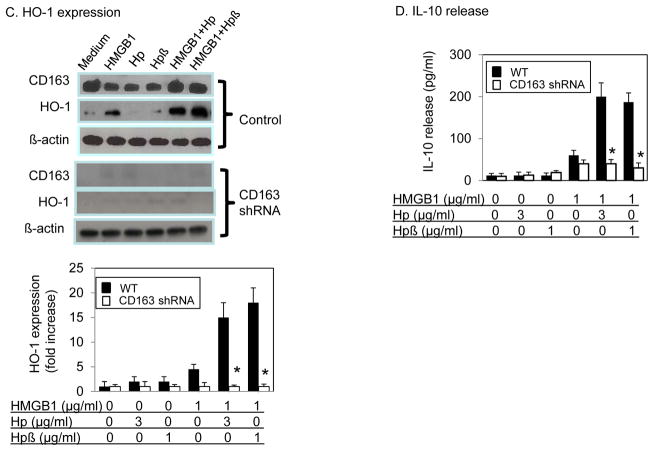
Figure 4. HMGB1 and haptoglobin (or β subunit) complexes signal through CD163 to induce HO-1 and IL-10 expression in macrophages.
(A) Surface plasmon resonance assay (SPR) of HMGB1 and haptoglobin β complexes to CD163. Recombinant human CD163 was coated on the sensor chip, the complexes of HMGB1 and haptoglobin β (1:1 molar ratio) at concentrations of 0, 32, 65, 125 or 250 nM were flow over the chip and the binding to CD163 (response units) was recorded. The Kd of HMGB1 and haptoglobin β complexes to CD163 is approximately 70 nM. Data are representative of 3 separate experiments.
(B) Human monocytic THP-1 cells (in 24-well plate) were cultured with dexamethasone (2.5×10−7 M) for 2 days to induce CD163 expression. Cells were then stimulated with HMGB1 alone (5 μg/ml) in combination with haptoglobin (15 μg/ml) or haptoglobin β (5 μg/ml) at 37°C for 16 hours. After incubation, cell cultures were centrifuged and supernatants were collected to measure IL-10 release. The expression of HO-1 and β-actin in cell lysate was measured by western blot. Data are expressed as folds of the unstimulated group after normalization to β-actin (n = 3 experiments). *: p<0.05 vs. HMGB1 alone.
(C–D) Knock down CD163 abolishes HMGB1 and haptoglobin (or haptoglobin β) complexes-induced HO-1 and IL-10 expression in macrophages. Primary human macrophages were transduced with specific shRNA lentiviral particles targeting CD163 or vector alone (control). At 72 hours after transduction, cells (in 24-well plate) were stimulated with HMGB1 (1 μg/ml) with or without haptoglobin (3 μg/ml) or haptoglobin β (1 μg/ml) for 16 hours. Cell cultures were centrifuged. The expression of CD163, HO-1 and β-actin in cell lysate was measured by western blot (C). Supernatants were collected to measure IL-10 release (D). Data are presented as folds of unstimulated group after normalization to β actin (n = 3 experiments). *: P<0.05 vs. WT.
(E) Residential peritoneal macrophages from CD163−/− and wild type control mice were stimulated with HMGB1 (1 μg/ml) alone, plus haptoglobin (3 μg/ml) or haptoglobin β (1 μg/ml) for 16 hours. IL-10 released and HO-1 expression in cell lysate were measured using ELISA kits (n = 3 experiments). *: P<0.05 vs. WT.