Abstract
Besides amyloidosis and light chain deposition disease, the most common histological type of renal lesion is cast nephropathy in 30% of patients with multiple myeloma [2]. In contrast to amyloidosis, cast nephropathy is believed to be potentially reversible when circulating light chains are rapidly reduced. We report on three patients with multiple myeloma and cast nephropathy treated with a bortezomib-based chemotherapy in addition to a newly developed high-cutoff polyflux® haemofilter. Reduction in serum free light chain levels was achieved within 10–12 days, with all three patients improving their renal function.
Keywords: cast nephropathy, free light chains, haemodialysis, high cutoff, myeloma
Introduction
Renal involvement in myeloma affects up to 50% of patients at initial presentation [1]. The most common histological type of renal lesion is cast nephropathy in 30% of patients with multiple myeloma [2]. Cast nephropathy develops after glomerular filtration of free light chains, subsequent tubular precipitation of light chains together with other proteins and mechanical obstruction. Interstitial inflammation caused by excessive reabsorption of light chains might contribute to renal damage. To remove light chains in patients with myeloma and acute renal failure, several studies were performed using plasma exchange [3,4]. Most recently, the use of high-cutoff haemodialysis membranes has been reported to effectively remove circulating light chains [5], but together with various combined chemotherapies only 5 of 13 patients became dialysis-independent [5]. Higher rates of complete remission (18–32%) compared to conventional chemotherapy have been reported for induction therapy with the proteasome inhibitor bortezomib [6,7]. We therefore speculated that first-line therapy with bortezomib combined with extracorporeal therapy using high-cutoff filters is even more efficacious in reducing levels of light chains, possibly improving acute renal failure in cast nephropathy.
Material and methods
High-cutoff haemodialysis
High-cutoff therapy was performed using a newly developed polyflux® haemodialyzer (HCO1100; Gambro Dialysatoren, Hechingen, Germany). The haemodialyzer is designed to increase permeability for substances in the molecular weight range up to 60 kD [8,9]. Treatment was performed daily for 4 h at blood flows of 250 to 300 ml/min until light chain levels were < ∼20% of initial levels. Regular dialysis machines and dialysate were used for this study. Plasma levels of free light chains were measured before and after most dialysis sessions by nephelometry (FREELITE, The Binding Site, Birmingham, UK [5]).
Combined chemotherapy
Bortezomib (Velcade®, 1.3 mg/m2) was administered by intravenous bolus on Days 1, 4, 8, and 11. Therapy was repeated on Days 22, 26, 30 and 33. In addition, the patients received 20 mg dexamethasone orally on the days when bortezomib was given as well as the day after. Cyclophosphamide was given orally at a dose of 50 mg/day.
Results and discussion
Case 1
A 66-year-old man presented with acute renal failure, progressive dyspnoe and pain in the lumbar spine. The bone marrow showed 80 to 90% infiltration with plasma cells; serum IgG was 4878 mg/dl and free immunoglobulin light chain lambda was determined at 4400 mg/l. The renal biopsy demonstrated cast nephropathy, intratubular cast obstruction and tubular toxicity. Serum creatinine was 4.5 mg/dl, and the glomerular filtration rate calculated by the MDRD-6 formula was 15 ml/min. Former serum creatinine levels were normal.
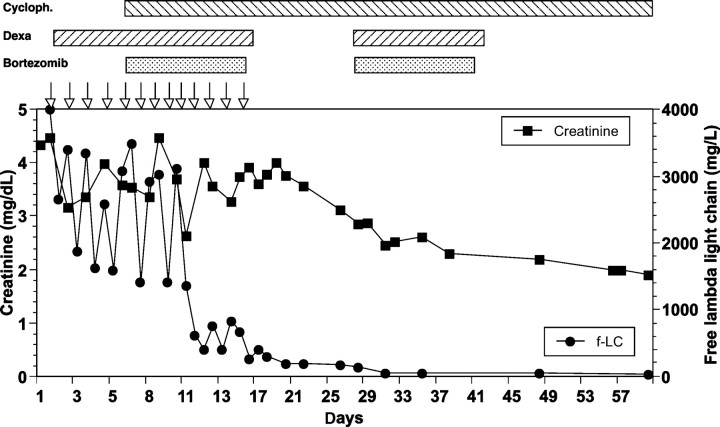
The initial treatment of daily haemodialysis using high-cutoff membranes and 40 mg dexamethasone per day was not sufficient to decrease free light chains in the blood persistently (Figure 1). After five days we started a combined, bortezomib-based chemotherapy and free light chains IgG in the blood decreased significantly.
Fig. 1.
Time course of serum creatinine and free light chains lambda for patient 1. Open arrows denote haemodialysis using high-cutoff dialyzers. Chemotherapy was given as indicated above the graph.
Approximately 10 days after starting therapy with high-cutoff haemofilter and chemotherapy renal function stabilized and we stopped haemodialysis. Four months after starting therapy, the GFR is >30 ml/min; serum free light chains and the kappa/lambda ratio are normalized and there is no proteinuria.
Case 2
A 63-year-old woman was admitted because of a rise in creatinine to 5.0 mg/dl (MDRD-GFR 10 ml/min). Three weeks before, the creatinine was 1.0 mg/dl. Except for back pain for the last 4 weeks, she was without symptoms. Diagnostic test revealed a monoclonal free light chain lambda of 3100 mg/l; the bone marrow biopsy showed diffuse infiltration with plasma cells up to 80%. Renal biopsy showed tubulo-interstitial nephritis and tubular casts positive for IgG lambda. After 7 days of treatment with dexamethasone and haemodialysis using high-cutoff membrane, levels of free light chains decreased significantly to ∼50%. When the results of the bone marrow specimen became available, we started combined chemotherapy as described above. Free light chains decreased further to normal levels. A total of six haemodialysis sessions were performed. After 2 months, the MDRD-GFR was 22 ml/min, and the free light chain lambda was 54 mg/l.
Case 3
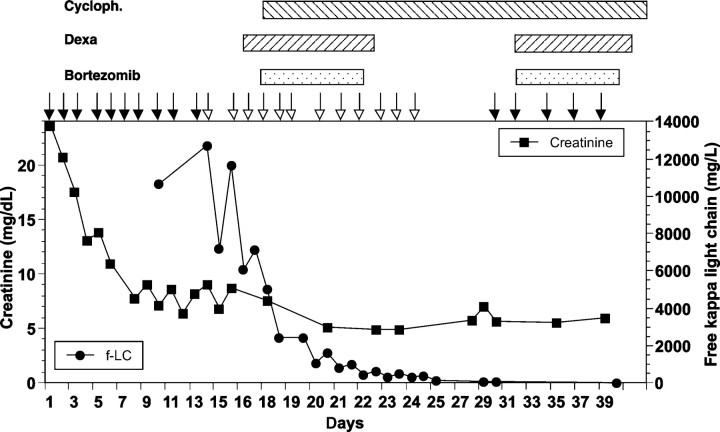
A 77-year-old male presented with fatigue and occasional disorientation. He was hypertensive but did not take medication regularly. His initial serum creatinine was 23 mg/dl, and urea-N was 140 mg/dl. Haemodialysis with regular dialyzers was initiated immediately (Figure 2). Levels of free light chain kappa were 10 700 mg/l, proteinuria was about 3 g/day. Renal biopsy revealed severe tubulo-interstitial nephritis and casts positive for kappa light chains. In addition, there were marked ischaemic glomerular lesions (9 sclerosed glomerula out of 20). The bone marrow biopsy showed only a slight increase in CD138-positive plasma cells making up 15 to 20% of the cellularity. The type of haemodialyzers was switched to high-cutoff membranes and combined chemotherapy was started 5 days later (Figure 2). However, the patient remained dialysis-dependent, but after 3 months, the haemodialysis frequency could be decreased to twice a week. There is ∼400 mg/day proteinuria.
Fig. 2.
Time course of serum creatinine and free light chains kappa in patient 3. Open arrows denote haemodialysis using high-cutoff dialyzers and closed arrows denote haemodialysis using regular dialyzers. Chemotherapy was given as indicated above the graph.
Discussion
By using medical treatment including bortezomib, cyclophosphamide and dexamethasone combined with extracorporeal removal of light chains we were able to rapidly lower serum levels of free light chains and improve renal function in three consecutive patients. In the first patient, dexamethasone plus high-cutoff haemofilters alone was not able to reduce light chain levels considerably (Figure 1); only after the introduction of bortezomib and cyclophosphamide could a rapid and sustained decrease in light chains be achieved. One of our three patients remained dialysis-dependent, but he presented with very high creatinine levels indicating ongoing renal damage for a longer period, possibly from hypertensive nephropathy in addition to cast nephropathy.
Several reports described renal recovery from cast nephropathy even months after initial presentation with renal failure [10]. However, early studies reported that <20% of patients presenting with renal failure become dialysis-independent [11]. The rapid recovery of renal function in our case series suggests that ongoing filtration of free light chains contributes to constant renal injury. A fast-acting anti-myeloma therapy should be combined with the use of high-cutoff dialyzers. In the present case series we were able to decrease light chains <0.5 g/l within 10 to 12 days after starting combined therapy as described above. Furthermore, bortezomib can be safely administered to the patient with severe renal impairment. Toxicity was only mild with thrombocytopenia WHO grade I in one patient, and infection WHO grade II (herpes zoster reactivation) in another patient after three cycles of chemotherapy. The combination of bortezomib-based therapy and extracorporeal removal of light chains using high-cutoff dialyzers appears as hitherto the most efficient approach to decrease light chain levels. Direct comparisons between different combinations of chemotherapy with or without extracorporeal removal of light chains are needed to determine the optimal treatment of acute renal failure due to light chain nephropathy.
Conflict of interest statement. One of the authors (M.S.) is currently employed by Gambro. R.S. has received research support and honoraria from Gambro. The remaining authors declare no competing financial interests.
References
- 1.Knudsen LM, Hippe E, Hjorth M, et al. (The Nordic Myeloma Study Group) Renal function in newly diagnosed multiple myeloma–a demographic study of 1353 patients. Eur J Haematol. 1994;53:207–212. doi: 10.1111/j.1600-0609.1994.tb00190.x. [DOI] [PubMed] [Google Scholar]
- 2.Herrera GA, Joseph L, Gu X, et al. Renal pathologic spectrum in an autopsy series of patients with plasma cell dyscrasia. Arch Pathol Lab Med. 2004;128:875–879. doi: 10.5858/2004-128-875-RPSIAA. [DOI] [PubMed] [Google Scholar]
- 3.Clark WF, Stewart AK, Rock GA, et al. Plasma exchange when myeloma presents as acute renal failure: a randomized, controlled trial. Ann Intern Med. 2005;143:777–784. doi: 10.7326/0003-4819-143-11-200512060-00005. [DOI] [PubMed] [Google Scholar]
- 4.Zuchelli P, Pasquali S, Cagnali L, et al. Controlled plasma exchange trial in acute renal failure due to multiple myeloma. Kidney Int. 1988;33:1175–1180. doi: 10.1038/ki.1988.127. [DOI] [PubMed] [Google Scholar]
- 5.Hutchison CA, Cockwell P, Reid S, et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: in vitro and in vivo studies. J Am Soc Nephrol. 2007;18:886–895. doi: 10.1681/ASN.2006080821. [DOI] [PubMed] [Google Scholar]
- 6.Jagannath S. Bortezomib dosing in relapsed multiple myeloma. Clin Lymphoma Myeloma. 2006;7:101–102. doi: 10.3816/CLM.2006.n.045. [DOI] [PubMed] [Google Scholar]
- 7.Mateos MV, Hernandez JM, Hernandez MT, et al. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–2172. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 8.Morgera S, Haase M, Kuss T, et al. Pilot study on the effects of high cutoff hemofiltration on the need for norepinephrine in septic patients with acute renal failure. Crit Care Med. 2006;34:2099–2104. doi: 10.1097/01.CCM.0000229147.50592.F9. [DOI] [PubMed] [Google Scholar]
- 9.Morgera S, Slowinski T, Melzer C, et al. Renal replacement therapy with high-cutoff hemofilters: impact of convection and diffusion on cytokine clearances and protein status. Am J Kidney Dis. 2004;43:444–453. doi: 10.1053/j.ajkd.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Tauro S, Clark FJ, Duncan N, et al. Recovery of renal function after autologous stem cell transplantation in myeloma patients with end-stage renal failure. Bone Marrow Transplant. 2002;30:471–473. doi: 10.1038/sj.bmt.1703713. [DOI] [PubMed] [Google Scholar]
- 11.Pozzi C, D’Amico M, Fogazzi GB, et al. Light chain deposition disease with renal involvement: clinical characteristics and prognostic factors. Am J Kidney Dis. 2003;42:1154–1163. doi: 10.1053/j.ajkd.2003.08.040. [DOI] [PubMed] [Google Scholar]