Abstract
Purpose
In 2010, a randomized clinical trial demonstrated noninferior survival for patients with advanced ovarian cancer who were treated with neoadjuvant chemotherapy (NACT) compared with primary cytoreductive surgery (PCS). We examined the use and effectiveness of NACT in clinical practice.
Patients and Methods
A multi-institutional observational study of 1,538 women with stages IIIC to IV ovarian cancer who were treated at six National Cancer Institute–designated cancer centers. We examined NACT use in patients who were diagnosed between 2003 and 2012 (N = 1,538) and compared overall survival (OS), morbidity, and postoperative residual disease in a propensity-score matched sample of patients (N = 594).
Results
NACT use increased from 16% during 2003 to 2010 to 34% during 2011 to 2012 in stage IIIC disease (Ptrend < .001), and from 41% to 62% in stage IV disease (Ptrend < .001). Adoption of NACT varied by institution, from 8% to 30% for stage IIIC disease (P < .001) and from 27% to 61% (P = .007) for stage IV disease during this time period. In the matched sample, NACT was associated with shorter OS in stage IIIC disease (median OS: 33 v 43 months; hazard ratio [HR], 1.40; 95% CI, 1.11 to 1.77) compared with PCS, but not stage IV disease (median OS: 31 v 36 months; HR, 1.16; 95% CI, 0.89 to 1.52). Patients with stages IIIC and IV disease who received NACT were less likely to have ≥ 1 cm postoperative residual disease, an intensive care unit admission, or a rehospitalization (all P ≤ .04) compared with those who received PCS treatment. However, among women with stage IIIC disease who achieved microscopic or ≤ 1 cm postoperative residual disease, NACT was associated with decreased OS (HR, 1.49; 95% CI, 1.01 to 2.18; P = .04).
Conclusion
Use of NACT increased significantly between 2003 and 2012. In this observational study, PCS was associated with increased survival in stage IIIC, but not stage IV disease. Future studies should prospectively consider the efficacy of NACT by extent of residual disease in unselected patients.
INTRODUCTION
Optimal treatment of advanced ovarian cancer includes both surgical cytoreduction and platinum-based chemotherapy. In 2010, a randomized clinical trial (RCT) demonstrated that women with stages IIIC and IV ovarian cancer who received neoadjuvant chemotherapy (NACT) had noninferior progression-free survival (PFS) and overall survival (OS) and less treatment-related morbidity and mortality compared with those who received primary cytoreductive surgery (PCS).1 More recently, another RCT reported that NACT was noninferior to PCS.2 Both trials have been criticized because the median overall survival, mean operative times, and rates of optimal cytoreduction were significantly lower than expected.
To date, few studies have examined the impact that these trials have had on the use of NACT in clinical practice, particularly in the United States, where the choice between PCS and NACT remains highly controversial. Two meta-analyses examined outcomes of NACT, but reported opposing findings.3,4
In this study, we examined the use of NACT between 2003 and 2012 at six comprehensive cancer centers as well as outcomes associated with NACT, including: OS, treatment-related morbidity, and surgical outcomes, including postoperative residual disease. We hypothesized that use of NACT would increase significantly after publication of the first RCT and would be associated with decreased perioperative morbidity compared with PCS. We further hypothesized that women who received NACT and interval cytoreductive surgery (ICS) would be more likely to have microscopic or ≤ 1 cm disease postoperatively compared with PCS, but would experience comparable OS.
MATERIALS AND METHODS
Data Source
The National Comprehensive Cancer Center Network (NCCN) Ovarian Cancer Outcomes Database Project was created in 2005. Between October 2005 and June 2012, data were prospectively collected on all patients who were diagnosed with ovarian, fallopian, or primary peritoneal cancers at six institutions: City of Hope Comprehensive Cancer Center, Dana-Farber/Brigham and Women’s Cancer Center, Fox Chase Cancer Center, Ohio State University Comprehensive Cancer Center, The University of Texas MD Anderson Cancer Center, and the University of Michigan Comprehensive Cancer Center. Data from October 2003 and September 2005 were abstracted retrospectively. Patients seen for one consultation were excluded.
Data on patient and disease characteristics, surgical procedures, postoperative residual disease, chemotherapy, and vital status were abstracted from medical records from diagnosis to death for all patients receiving ongoing care at participating centers. Vital status was confirmed in the National Death Index and medical records abstraction. The institutional review board at each center approved the overall project, and the Dana-Farber/Harvard Cancer Center’s Office for Human Research Studies deemed this study exempt from review.
Study Cohorts
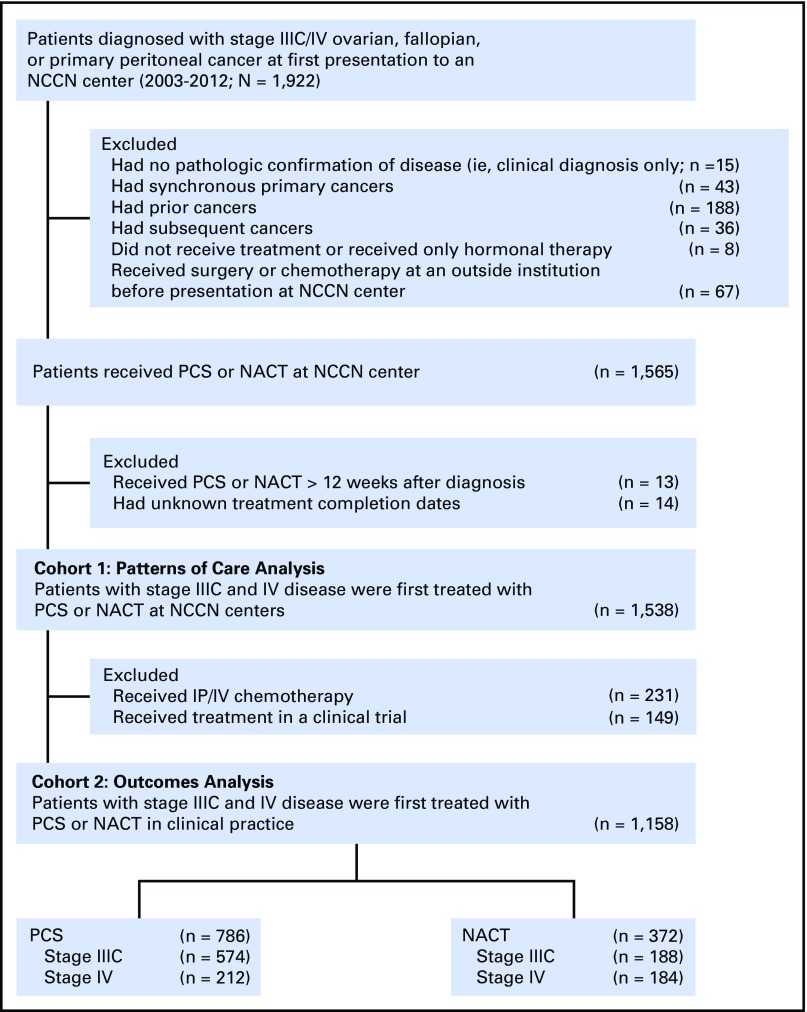
We identified all patients who were diagnosed with stages IIIC and IV ovarian cancer between 2003 and 2012 who received NACT or PCS at an NCCN center. Patients were assigned to a treatment arm (NACT or PCS) on the basis of whether they first received chemotherapy or surgery. In cohort 1, we examined NACT use over time among 1,538 patients diagnosed between 2003 and 2012 and treated within 12 weeks of diagnosis. In cohort 2, we examined factors and outcomes associated with NACT versus PCS within a subset of 1,158 patients from cohort 1, excluding patients who received intraperitoneal and intravenous (IP/IV) chemotherapy—as few patients treated with NACT receive IP/IV chemotherapy, and it is associated with an independent survival benefit5,6—and clinical trial participants to minimize selection bias.7 The flow diagram is displayed in Appendix Figure A1 (online only).
Outcomes
Our primary outcomes of interest were the proportion of patients who received NACT over time and OS. Survival time started at the first dose of NACT or date of PCS. Patient deaths were abstracted through May 20, 2016; patients alive after this date were censored. Secondary outcomes included treatment-related morbidity and surgical outcomes, that is, residual disease, aggressiveness of surgery, and surgical complexity score.8
Independent Variables
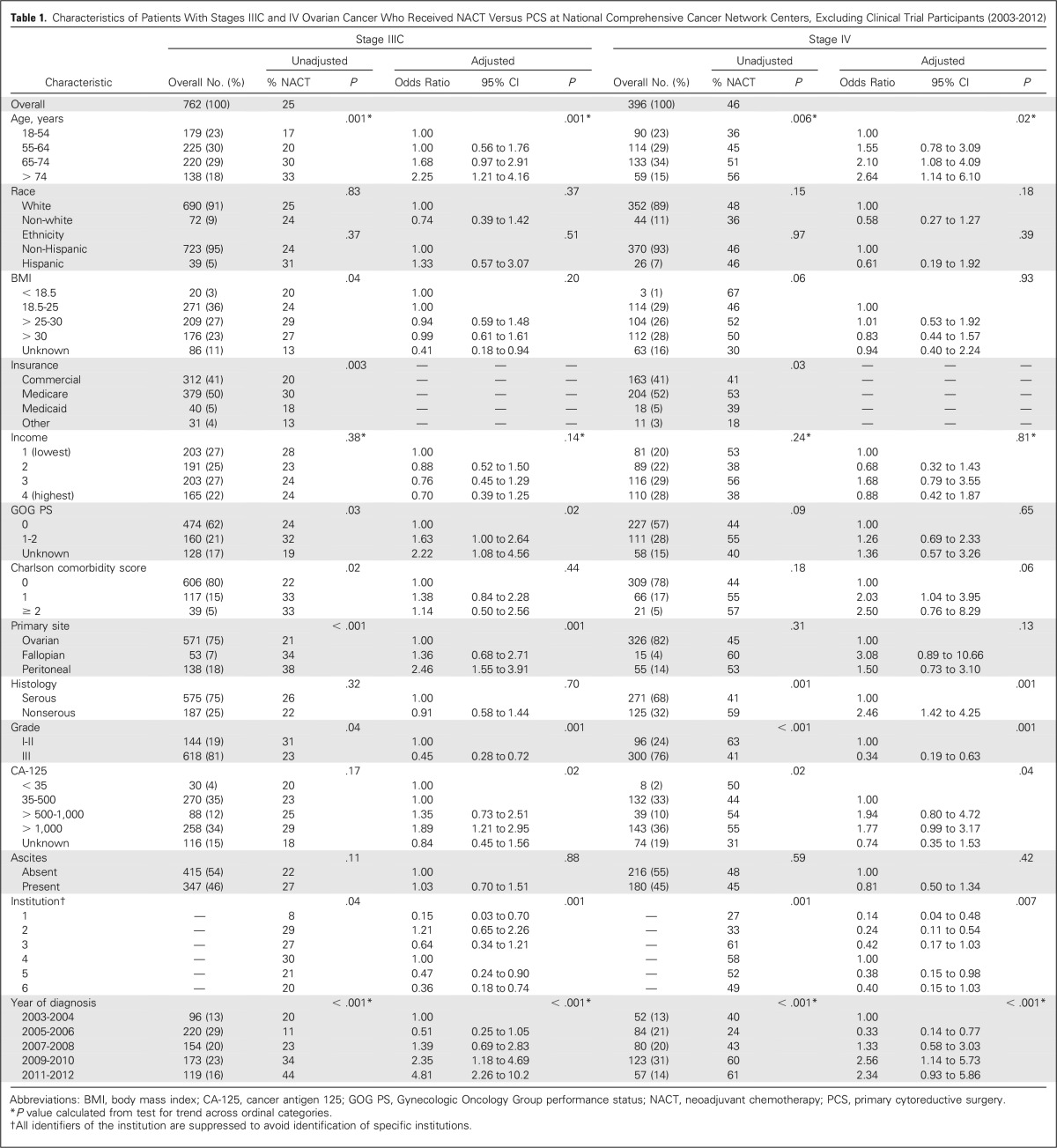
The primary independent variable of interest for the analysis examining NACT use over time was year of diagnosis. For outcomes analysis, the primary predictor was NACT or PCS. Covariates obtained from the medical record included age at diagnosis, race/ethnicity, pretreatment body mass index, insurance, income, Gynecologic Oncology Group performance status, Charlson comorbidity score,9 primary disease site, histology, grade, baseline cancer antigen 125 (CA-125), presence of ascites, and NCCN institution.
Analyses
All analyses were conducted separately by stage. In cohort 1, we tested for changes in the proportion of patients receiving NACT over time by using the Cochran-Armitage test for trend. In cohort 2, we used multivariable logistic regression to investigate factors associated with NACT, including all independent variables of interest regardless of statistical significance, except for insurance as a result of its collinearity with age; outcomes analyses were conducted in propensity-score matched cohorts to balance covariates that might confound the effect of treatment approach on survival.10-12 Patients who received NACT were matched to patients who received PCS by nearest neighbor matching using a caliper of 0.2 of the pooled standard deviation of the log odds of the propensity score, which was calculated by using all variables in Table 1.
Table 1.
Characteristics of Patients With Stages IIIC and IV Ovarian Cancer Who Received NACT Versus PCS at National Comprehensive Cancer Network Centers, Excluding Clinical Trial Participants (2003-2012)
Within the propensity-score matched cohort, survival curves were estimated by using Kaplan-Meier methods. A Cox proportional hazards regression model was used to examine associations between primary treatment (NACT v PCS) and OS, with a robust variance estimator to correct for clustering within matched pairs. Conditional logistic regression for matched groups was used to assess associations between primary treatment and treatment-related morbidity and surgical outcomes. To confirm that this analysis was not impacted by immortal time bias, which we thought would be minimal because both groups received some form of treatment within 12 weeks after diagnosis, we repeated the survival analysis for matched pairs who survived to a landmark time of 3 months after diagnosis.
Although we adjusted for a large number of observed patient and disease characteristics by using propensity score analyses, these methods cannot adjust for unmeasured confounders. Therefore, we tested the sensitivity of associations between treatment group and OS to an unmeasured confounder: high disease burden. By using data from the GOG-182 study to estimate the effect of preoperative disease burden on OS, we estimated that women with high preoperative disease burden have a hazard ratio (HR) of death of 1.56 compared with women with a moderate or low disease burden.13 We instituted a range of assumptions about the prevalence of high disease burden, which is likely to be more prevalent in women who receive NACT.
We performed several sensitivity analyses. First, we repeated all analyses after including women who received IP/IV chemotherapy (n = 34). Second, we limited the survival analysis to matched pairs with patients treated with NACT who received ICS to exclude patients who may have received chemotherapy with strictly palliative intent. Third, we examined associations between treatment and OS within the subgroup of patients with stage IIIC disease and either R0 resection or ≤ 1 cm of residual disease. For each of these analyses, we refit the propensity score model by using the variables described above. Finally, we excluded institutions from the survival model one by one to ensure that the results were not explained by observations at a single institution.
Statistical tests were two sided and differences were considered statistically significant at P < .05. All statistical analysis was performed by using Stata software (version 13.1; Stata, College Station, TX; Computing Resource Center, Santa Monica, CA).
RESULTS
Use of NACT Over Time
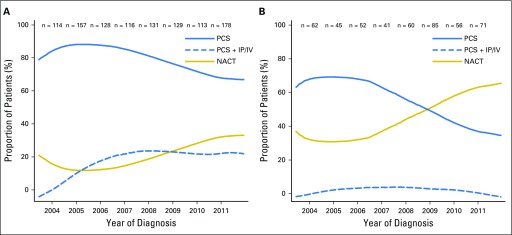
Overall, 206 (19%) of 1,066 patients with stage IIIC disease and 210 (44%) of 472 patients with stage IV ovarian cancer received NACT between 2003 and 2012. Figure 1 shows the use of NACT versus PCS with and without IP/IV chemotherapy over time (cohort 1). In women with stage IIIC disease, NACT use increased from 16% during 2003 to 2010 to 34% during 2011 to 2012 (Ptrend < .001). For women with stage IV disease, NACT use increased from 41% during 2003 to 2010 to 62% during 2011 to 2012 (Ptrend < .001).
Fig 1.
(A) Stage IIIC disease. (B) Stage IV disease. Use of neoadjuvant chemotherapy (NACT) increased significantly over time (Ptrend < .001 for both groups). Intraperitoneal and intravenous (IP/IV) chemotherapy is shown for comparison. Three patients with stage IIIC disease and one with stage IV who were diagnosed in 2003 are included in the estimate for 2004. Twenty-three patients with stage IIIC disease and seven with stage IV who were diagnosed in 2012 are included in the estimate for 2011. PCS, primary cytoreductive surgery.
Factors Associated With NACT Use
Table 1 shows the characteristics of patients who received NACT versus PCS for stages IIIC and IV ovarian cancer, excluding clinical trial participants and those who received IP/IV chemotherapy (cohort 2). In adjusted analyses, patients with stage IIIC were more likely to receive NACT, compared with PCS, if they were older, had a higher performance status, or had a preoperative CA-125 of > 1,000 (all P ≤ .02); in patients with stage IV disease, age and CA-125 were significantly associated with NACT use (P ≤ .04). Use of NACT varied significantly by institution, with an unadjusted range of 8% to 30% in stage IIIC disease (P < .001) and 27% to 61% in stage IV disease (P = .007).
Treatments Received
In cohort 2, 188 (25%) of 762 patients with stage IIIC disease and 184 (46%) of 396 patients with stage IV disease received NACT (Appendix Table A1, online only). Among patients who received NACT, 27 (14%) of 188 with stage IIIC and 53 (29%) of 184 with stage IV disease did not receive ICS. Among patients who received PCS, 54 (9%) of 574 patients with stage IIIC and 16 (8%) of 212 with stage IV disease did not receive postoperative chemotherapy.
Survival Outcomes
Propensity-score matching between NACT and PCS groups yielded 174 (93%) of 188 matched pairs for patients with stage IIIC ovarian cancer who received NACT and 123 (67%) of 184 matched pairs for patients with stage IV disease (Appendix Table A2, online only); standardized differences were < 10% for all variables (Appendix Table A3, online only). Median follow-up for survivors was 61 months and 70 months, respectively, for patients with stage IIIC and IV disease.
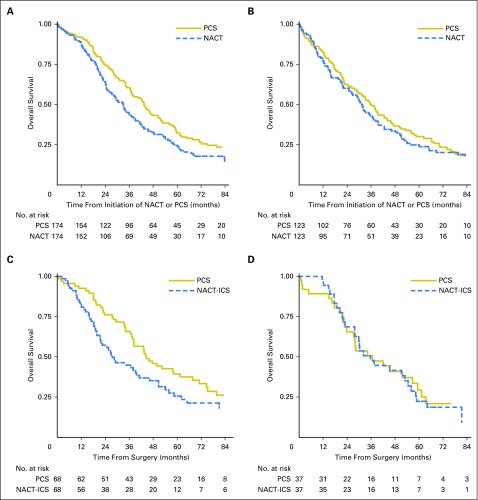
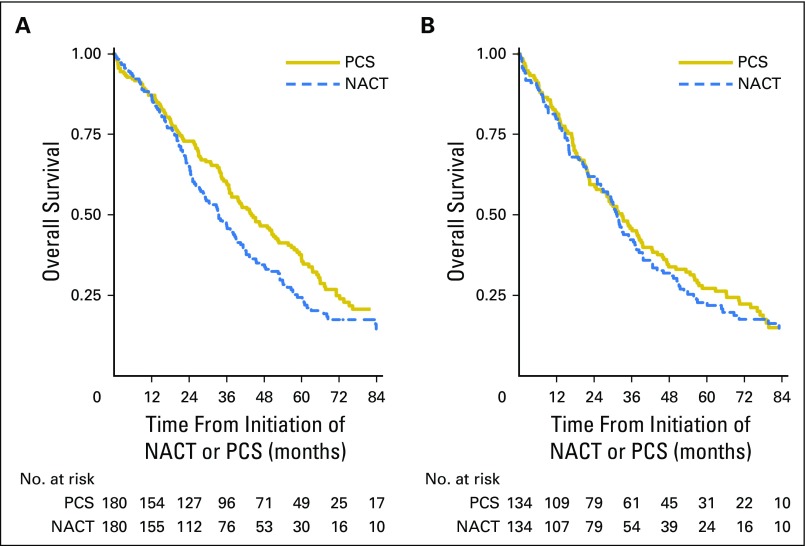
Patients with stage IIIC disease who received NACT had significantly decreased OS compared with those treated with PCS (median, 33 months v 43 months; HR, 1.40; 95% CI, 1.11 to 1.77; Fig 2A and Table 2). However, there was no significant difference in OS among patients with stage IV disease (median, 31 months v 36 months; HR, 1.16; 95% CI, 0.89 to 1.52; Fig 2B).
Fig 2.
Kaplan-Meier survival plots within propensity-score matched samples, separately by treatment with neoadjuvant chemotherapy (NACT) versus primary cytoreductive surgery (PCS). (A) Stage IIIC disease. (B) Stage IV disease. (C) Stage IIIC disease, ≤ 1 cm or optimal. (D) Stage IIIC disease, R0 resection. Panels C and D are limited to matched pairs who received interval cytoreductive surgery (NACT-ICS) versus PCS.
Table 2.
Results of Cox Proportional Hazards Regression Model for the Association Between NACT and PCS and Overall Survival Within Propensity-Score Matched Samples
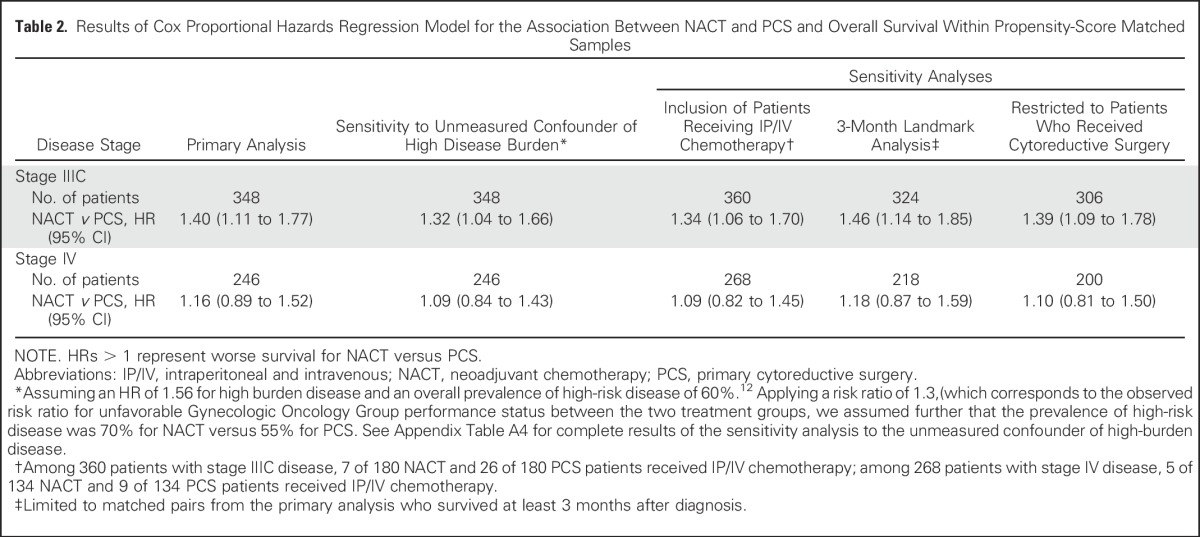
Survival outcomes were robust to sensitivity analyses, which tested associations between NACT and OS with the addition of an unmeasured confounder—high disease burden—receipt of IP/IV chemotherapy, a 3-month landmark analysis, and a cohort that excluded patients who received NACT without ICS (Table 2). Our results were robust to relatively large differences in the prevalence of high disease burden. For example, given evidence that high preoperative disease burden is associated with higher mortality (HR, 1.56) compared with moderate or low burden disease,13 if 80% of women who received NACT and 60% of women who received PCS had high burden disease, then the association between PCS and increased OS was still significant (Appendix Table A4, online only). There were few differences in the models that included patients who received IP/IV chemotherapy (Table 2 and Appendix Fig A2, online only).
In a subset analysis performed to compare survival outcomes stratified by residual disease, we found that NACT was associated with significantly decreased OS within the subgroup of patients who had stage IIIC disease and ≤ 1 cm residual disease after surgery (HR, 1.49; 95% CI, 1.01 to 2.18; P = .04) compared with PCS (Fig 2C). In this group, median survival was 45 months in patients who received PCS compared with 28 months in patients who received NACT. Among the small subset of patients who achieved no gross residual disease (n = 74), survival was similar between the two treatment groups (HR, 1.08; 95% CI, 0.69 to 1.69; Fig 2D). Finally, adjusted HRs for NACT versus PCS ranged from 1.31 to 1.53 for stage IIIC disease and from 1.03 to 1.28 for stage IV disease upon excluding institutions one by one, which indicated that our findings were not driven by outcomes at a single institution.
Surgical Morbidity Outcomes
As shown in Table 3, there were no significant differences in 28-day mortality, venous thrombotic events, cardiac complications, or respiratory failure between patients with stage IIIC disease who received PCS or NACT. In adjusted analyses, patients who received NACT were less likely to be admitted to an intensive care unit (ICU; 1.1% v 8.0%; P = .01) or rehospitalized (4.6% v 12.1%; P = .01) compared with those who received PCS. As shown in Table 4, results were similar among patients with stage IV disease. In the subset of patients who received NACT without ICS, 32% of patients with stage IV disease and 22% of patients with stage IIIC disease died within 28 days of their first cycle of NACT.
Table 3.
Comparison of Chemotherapy, Morbidity, and Surgical Outcomes Between Patients With Stage IIIC Ovarian Cancer Who Underwent NACT Versus PCS Treatment
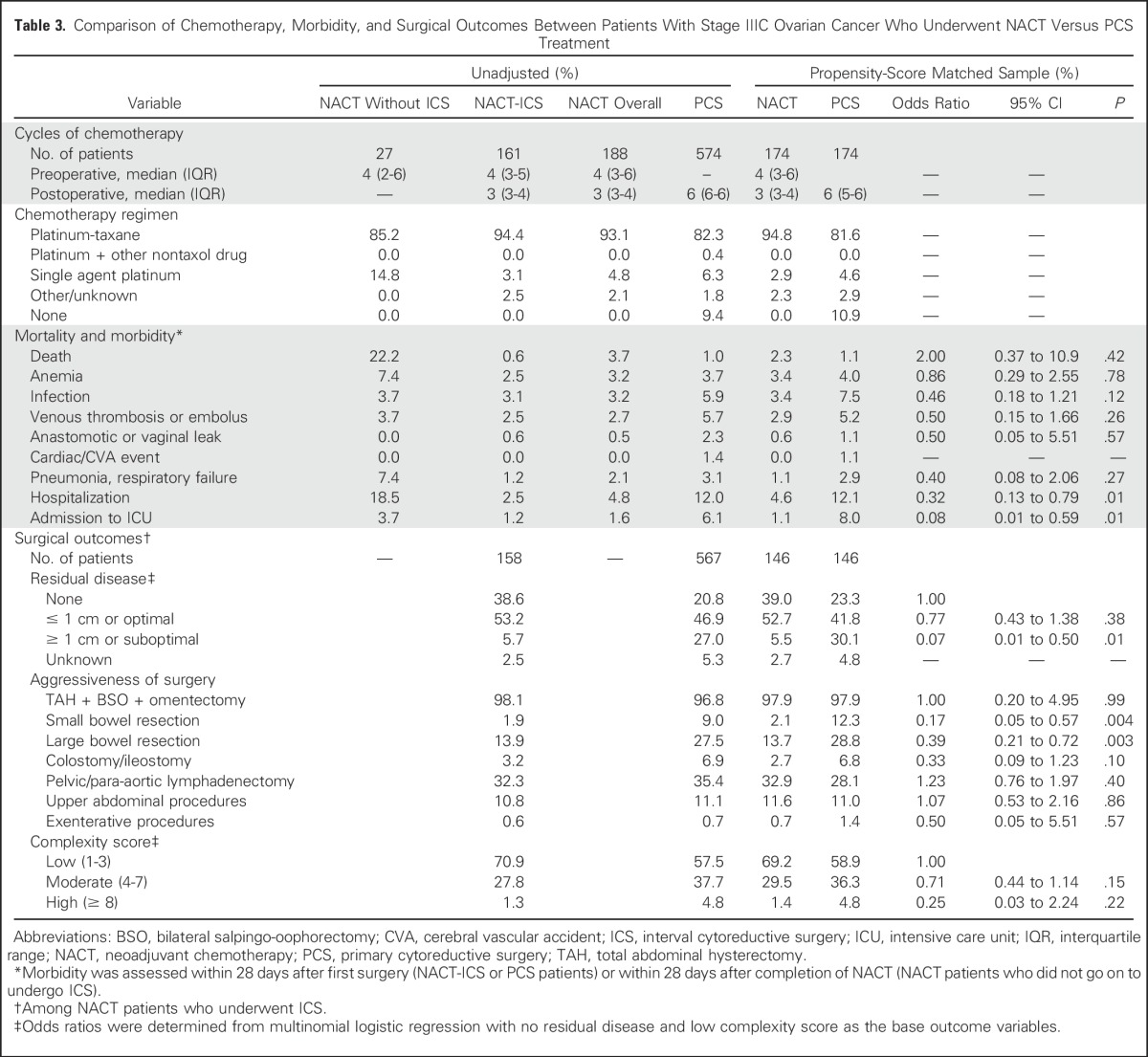
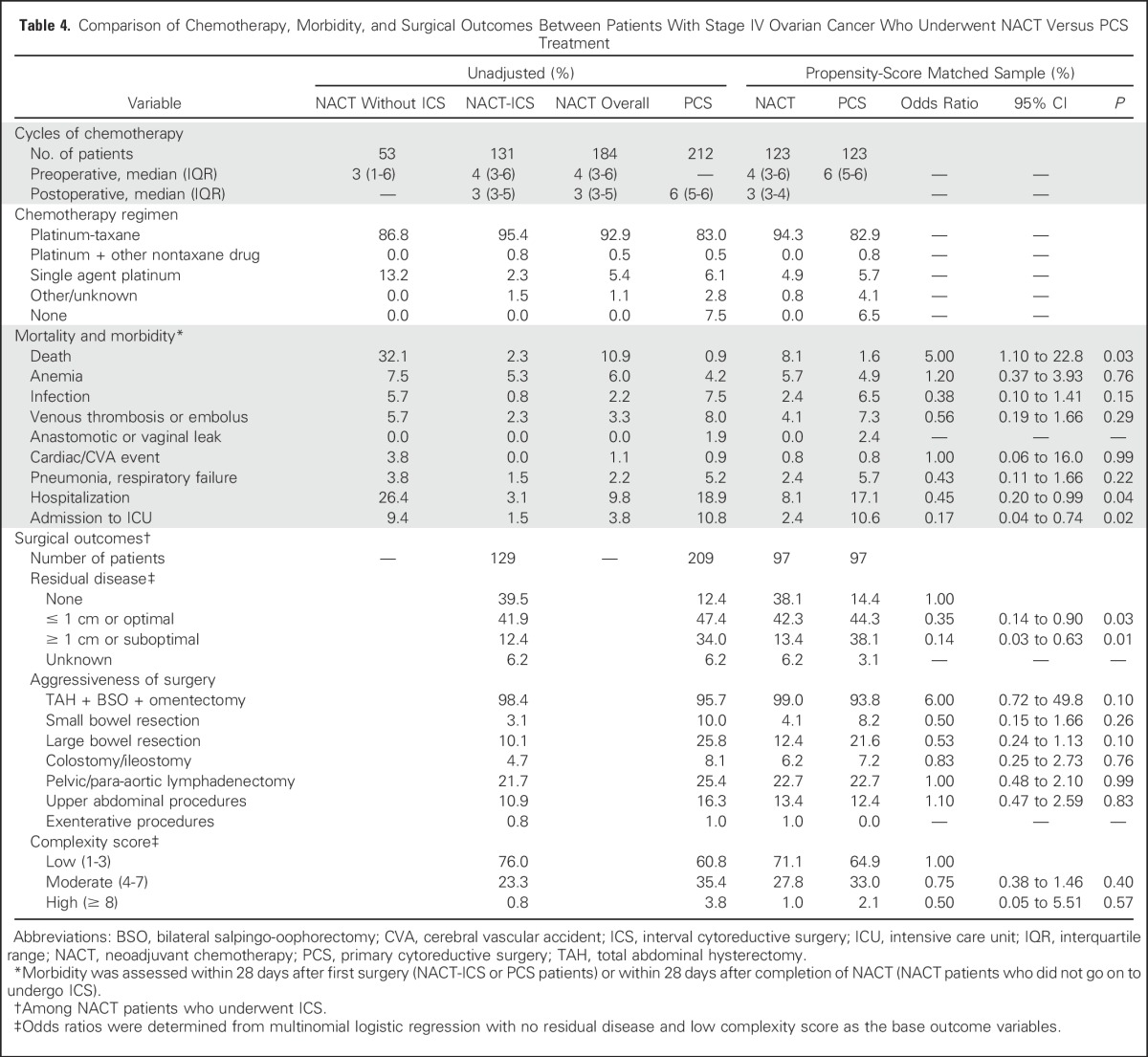
Table 4.
Comparison of Chemotherapy, Morbidity, and Surgical Outcomes Between Patients With Stage IV Ovarian Cancer Who Underwent NACT Versus PCS Treatment
Surgical Aggressiveness and Complexity Outcomes
Patients in the NACT group received a median of four cycles of chemotherapy before ICS and three cycles postoperatively, whereas patients in the PCS group received a median of six cycles of chemotherapy postoperatively (Tables 3 and 4). Those who received NACT were significantly less likely to have gross residual disease of ≥ 1 cm after ICS (stage IIIC: 5.5% v 30.1%; P = .01; stage IV: 13.4% v 38.1%; P = .01) compared with patients who underwent PCS.
Irrespective of stage, most patients who underwent NACT or PCS had low surgical complexity scores (Tables 3 and 4). In adjusted analyses, NACT was not significantly associated with lower complexity scores or decreased aggressiveness of surgery, that is, upper abdominal or exenterative procedures, ostomies. However, in patients with stage IIIC disease, NACT was associated with fewer bowel resections (small bowel: 2.1% v 12.3%; P = .004; large bowel: 13.7% v 28.8%; P = .003) compared with PCS. Similar associations were observed in patients with stage IV disease, although they did not reach statistical significance.
DISCUSSION
In this study of the use and effectiveness of NACT in patients with stages IIIC and IV ovarian cancer at six major cancer centers, we found that the adoption of NACT increased steadily over time from 16% to 34% (stage IIIC) and from 41% to 62% (stage IV) between 2003 and 2011. We also found that PCS was associated with significantly improved survival in women with stage IIIC ovarian cancer compared with NACT, but not in patients with stage IV disease. Patients who received NACT were more likely to achieve ≤ 1 cm or microscopic residual disease after ICS compared with PCS, but there were few differences in the complexity, aggressiveness, or complications of surgery. Of note, patients who received NACT were less likely to be admitted to an ICU or to be rehospitalized compared with patients who received PCS.
To our knowledge, this is one of the first, multi-institutional comparative effectiveness studies to demonstrate that PCS is associated with significantly improved survival among women with stage IIIC ovarian cancer. Although these results differ from the main findings of two large, prospective randomized trials, they are consistent with a subset analysis in the EORTC study which demonstrated improved survival in patients with stage IIIC ovarian cancer and ≤ 45 mm of disease in patients who received PCS versus NACT. Consistent with data from published RCTs, we observed no significant differences in survival between patients with stage IV disease who were treated with PCS and NACT.
Of note, although patients with stage IIIC disease who received NACT were significantly more likely to have ≤ 1 cm or R0 microscopic residual disease after ICS, this was not associated with a survival benefit. In contrast, patients who achieved ≤ 1 cm of residual disease after PCS had significantly longer survivals compared with comparable patients who received NACT. These findings suggest that optimal resection after ICS is not equivalent to PCS, perhaps because microscopic chemoresistant subclones may remain.14,15 Future studies should prospectively compare survival outcomes of patients treated with PCS versus NACT stratified by residual disease after surgery.
In prior studies, R0 resection at the time of PCS has been shown to be the single most important independent prognostic factor in advanced ovarian cancer,16-20 with a reported median survival of 110 months.21 These findings have led to a redefinition of the term optimal cytoreduction, from no residual mass > 1.0 cm to a new standard of complete resection to no visible residual disease.22,23 In our patient population, we did not observe a survival difference between patients who received NACT or PCS and who achieved an R0 resection. This may be a result of misclassification, that is, patients cytoreduced to no gross residual may have been categorized as optimal, as the importance between the distinction of an R0 resection and ≤ 1 cm residual was not widely recognized during the study period. In addition, this analysis was limited by a small sample size.
We also found fewer differences between PCS and NACT than expected in the morbidity, aggressiveness of surgery, and surgical complexity scores. Although fewer morbidities tended to be associated with NACT, there were few statistically significant differences. This may be because the overall surgical morbidity among all groups was low at these major academic centers.
The degree of variation in the use of NACT between such similar academic institutions was notable, particularly because patients’ sociodemographic and disease characteristics are relatively uniform across NCCN centers.24 These findings suggest that uptake of NACT is influenced by local culture and clinical practice leaders within institutions.25,26 Patients’ preferences may also have contributed.27
Our study has limitations that are inherent to all observational studies, including potential selection bias, as we were unable to control for unobserved confounds, for example, physician bias or operative effort, despite rigorous propensity-score analyses. In addition, the match rate for patients with stage IV disease was moderate (67%), although our results were robust to multiple sensitivity analyses. Despite these limitations, our study had many strengths, including the fact that it likely included a more representative population of patients than has traditionally been enrolled in prior RCTs that compared NACT and PCS treatment. In addition, we were able to adjust for many clinical characteristics that are rarely available in population-based databases, yet have important prognostic implications, for example, performance status, baseline CA-125, and extent of residual disease.21 In addition, our findings were robust to substantial differences in one potential confounder: high disease burden.
Another limitation was our inability to determine PFS accurately, although PFS may be more prone to bias than OS in clinical practice as it depends on other factors, for example, frequency of surveillance testing with CA-125 or computed tomography scans.28 Although we expect our results to be more generalizable than clinical trials, they may not be representative of clinical practice outside of academic institutions as most are high-volume hospitals, a factor associated with improved survival.29,30
In conclusion, our findings demonstrate that use of NACT at NCCN centers increased significantly over time. Although additional biases may persist despite propensity-score matching, our results suggest that in carefully selected patients with stage IIIC disease, PCS is associated with a survival advantage, with overall low rates of surgical morbidity. In contrast, for patients with stage IV disease, our results confirm that NACT is noninferior to PCS for survival, with fewer ICU admissions and rehospitalizations, which suggests that NACT may be preferable for patients with stage IV ovarian cancer.
ACKNOWLEDGMENT
We acknowledge the following people for their contributions to this project: Jane C. Weeks, who was involved in the conception of this study and design of the database, but tragically did not live to see its completion; all of the study participants; the Ovarian Cancer Outcomes Consortium for material and administrative support; Janet Files and Dana Milne for administrative and organizational assistance; Layla Rouse and Monir Corona for assistance with development of the study database; and the National Comprehensive Cancer Network for providing support for the outcomes database infrastructure and management.
Appendix
Fig A1.
Study cohort. IP/IV, intraperitoneal and intravenous; NACT, neoadjuvant chemotherapy; NCCN, National Comprehensive Cancer Network; PCS, primary cytoreductive surgery.
Fig A2.
Sensitivity analysis including patients who received intraperitoneal and intravenous chemotherapy: Kaplan-Meier survival plots within propensity-score matched samples. (A) Stage IIIC disease. (B) Stage IV disease. NACT, neoadjuvant chemotherapy; PCS, primary cytoreductive surgery.
Table A1.
Summary of Treatments Received
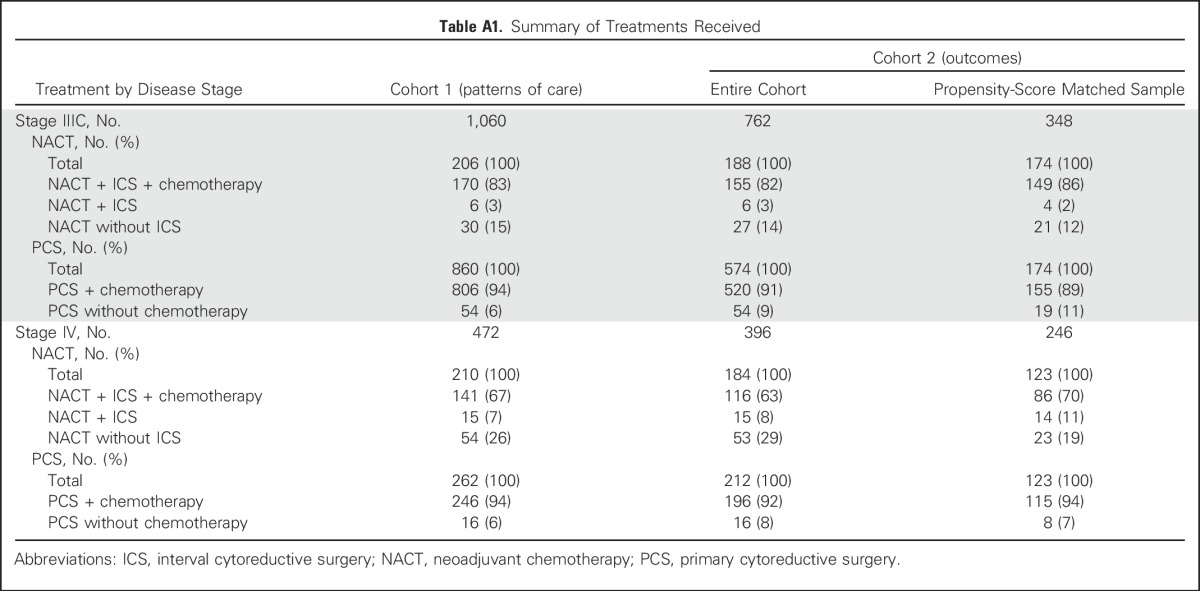
Table A2.
Patient Characteristics Within Propensity-Score Matched Samples
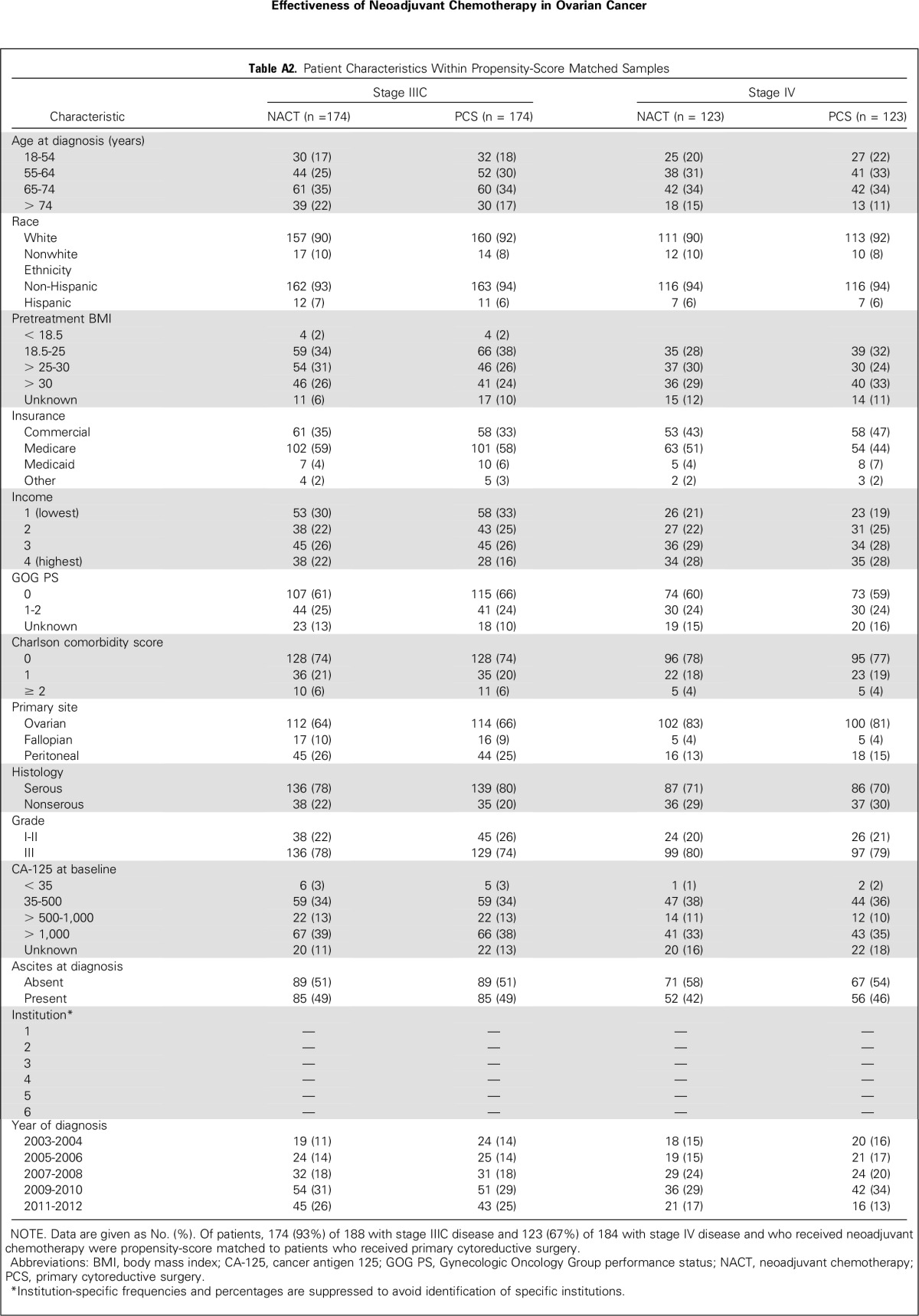
Table A3.
Standardized Difference in Observed Covariates Before and After Propensity-Score Matching
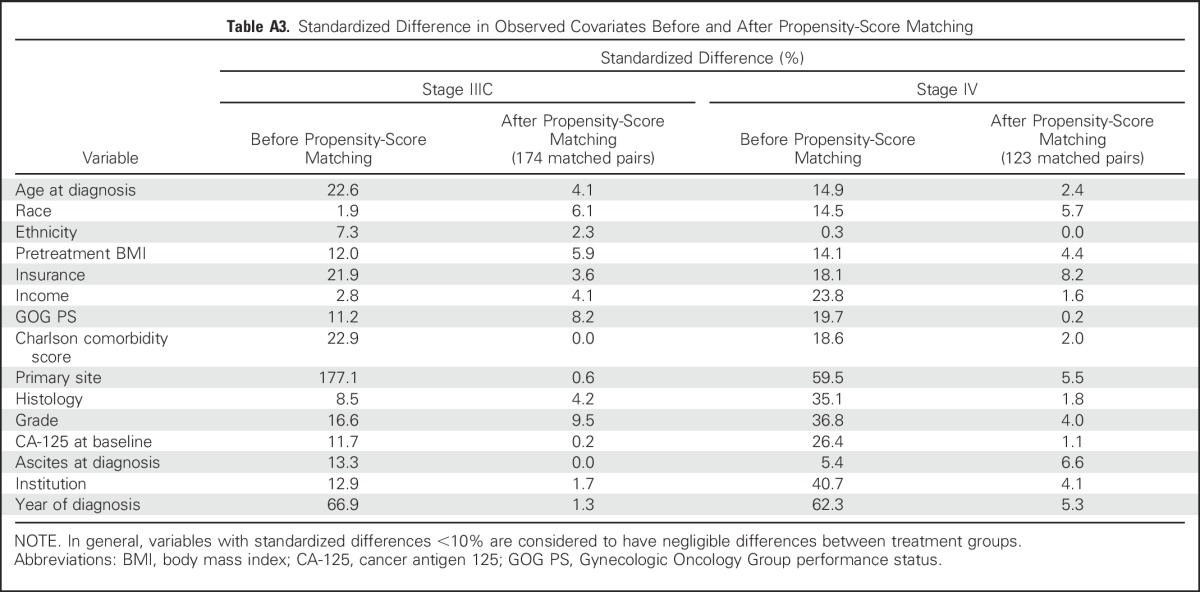
Table A4.
Sensitivity Analysis: HRs of NACT Versus PCS for Overall Survival, Adjusted for the Unobserved Confounder Variable of Disease Score
Footnotes
Supported by National Cancer Institute Grants No. K07 CA166210 (A.A.W.) and K07 CA201013 (L.A.M.) and Cancer Prevention and Research Institute of Texas Grant No. RP140020 (L.A.M.).
Presented in part at the 2015 Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 29-June 2, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
The funding organizations had no role in the preparation, review, or approval of the manuscript. A.A.W. and A.M.C. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
See accompanying editorial on page 3827
AUTHOR CONTRIBUTIONS
Conception and design: Larissa A. Meyer, Angel M. Cronin, Charlotte C. Sun, Alexi A. Wright
Financial support: Larissa A. Meyer, Alexi A. Wright
Administrative support: Larissa A. Meyer, Alexi A. Wright
Provision of study materials or patients: Larissa A. Meyer, Jennifer J. Griggs, Mihaela C. Cristea, Robert A. Burger, Ursula A. Matulonis, David M. O'Malley, Alexi A. Wright
Collection and assembly of data: Larissa A. Meyer, Charlotte C. Sun, Kristin Bixel, Mihaela C. Cristea, Jennifer J. Griggs, Charles F. Levenback, Gina Mantia-Smaldone, Ursula A. Matulonis, Joyce C. Niland, David M. O'Malley, Alexi A. Wright
Data analysis and interpretation: Larissa A. Meyer, Angel M. Cronin, Charlotte C. Sun, Kristin Bixel, Michael A. Bookman, Mihaela C. Cristea, Jennifer J. Griggs, Robert A. Burger, Ursula A. Matulonis, Joyce C. Niland, David M. O'Malley, Alexi A. Wright
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Larissa A. Meyer
Honoraria: TRM Oncology
Research Funding: AstraZeneca
Angel M. Cronin
No relationship to disclose
Charlotte C. Sun
Research Funding: AstraZeneca
Kristin Bixel
No relationship to disclose
Michael A. Bookman
Employment: McKesson
Honoraria: Genentech
Consulting or Advisory Role: Boehringer Ingelheim, Genentech, AstraZeneca, AbbVie, Sanofi, Novartis, Immunogen, Endocyte, Gradalis, Oxigene, Vertex, Pfizer, Clovis Oncology, Tesaro
Mihaela C. Cristea
Honoraria: Boehringer Ingelheim
Consulting or Advisory Role: Boehringer Ingelheim
Research Funding: Trovagene
Jennifer J. Griggs
No relationship to disclose
Charles F. Levenback
No relationship to disclose
Robert A. Burger
Consulting or Advisory Role: Genentech, Janssen Research & Development, NuCana BioMed, Gradalis, InVitae, Morphotek
Gina Mantia-Smaldone
No relationship to disclose
Ursula A. Matulonis
Consulting or Advisory Role: Merck KGaA, AstraZeneca, Immunogen, Genentech, Pfizer
Joyce C. Niland
No relationship to disclose
David M. O'Malley
Honoraria: Clovis Oncology
Consulting or Advisory Role: Janssen Oncology, Eisai, AstraZeneca, Clovis Oncology, Genentech
Research Funding: Amgen (Inst), VentiRx (Inst), AstraZeneca (Inst), Genentech (Inst), Regeneron (Inst), Immunogen (Inst), Array BioPharma (Inst), Janssen Research & Development (Inst), Clovis Oncology (Inst), EMD Serono (Inst), Ergomed (Inst), Ajinomoto (Inst), Cerulean Pharma (Inst), PharmaMar (Inst), Stem CentRx (Inst)
Alexi A. Wright
No relationship to disclose
REFERENCES
- 1.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: A meta-analysis. Gynecol Oncol. 2006;103:1070–1076. doi: 10.1016/j.ygyno.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 4.Kang S, Nam BH. Does neoadjuvant chemotherapy increase optimal cytoreduction rate in advanced ovarian cancer? Meta-analysis of 21 studies. Ann Surg Oncol. 2009;16:2315–2320. doi: 10.1245/s10434-009-0558-6. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 6.Wright AA, Cronin A, Milne DE, et al. Use and effectiveness of intraperitoneal chemotherapy for treatment of ovarian cancer. J Clin Oncol. 2015;33:2841–2847. doi: 10.1200/JCO.2015.61.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unger JM, Barlow WE, Martin DP, et al. Comparison of survival outcomes among cancer patients treated in and out of clinical trials. J Natl Cancer Inst. 2014;106:dju002. doi: 10.1093/jnci/dju002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aletti GD, Dowdy SC, Podratz KC, et al. Relationship among surgical complexity, short-term morbidity, and overall survival in primary surgery for advanced ovarian cancer. Am J Obstet Gynecol. 2007;197:676.e1-7. doi: 10.1016/j.ajog.2007.10.495. [DOI] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Rosenbaum P, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79:516–524. [Google Scholar]
- 12.Haukoos JS, Lewis RJ. The propensity score. JAMA. 2015;314:1637–1638. doi: 10.1001/jama.2015.13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz NS, Miller A, Rungruang B, et al. Does aggressive surgery improve outcomes? Interaction between preoperative disease burden and complex surgery in patients with advanced-stage ovarian cancer: An analysis of GOG 182. J Clin Oncol. 2015;33:937–943. doi: 10.1200/JCO.2014.56.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarz RF, Ng CK, Cooke SL, et al. Spatial and temporal heterogeneity in high-grade serous ovarian cancer: A phylogenetic analysis. PLoS Med. 2015;12:e1001789. doi: 10.1371/journal.pmed.1001789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke SL, Ng CK, Melnyk N, et al. Genomic analysis of genetic heterogeneity and evolution in high-grade serous ovarian carcinoma. Oncogene. 2010;29:4905–4913. doi: 10.1038/onc.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zivanovic O, Eisenhauer EL, Zhou Q, et al. The impact of bulky upper abdominal disease cephalad to the greater omentum on surgical outcome for stage IIIC epithelial ovarian, fallopian tube, and primary peritoneal cancer. Gynecol Oncol. 2008;108:287–292. doi: 10.1016/j.ygyno.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Aletti GD, Dowdy SC, Podratz KC, et al. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecol Oncol. 2006;100:283–287. doi: 10.1016/j.ygyno.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: A meta-analysis. J Clin Oncol. 2002;20:1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 19.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Polterauer S, Vergote I, Concin N, et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: Analysis of the OVCAD data. Int J Gynecol Cancer. 2012;22:380–385. doi: 10.1097/IGC.0b013e31823de6ae. [DOI] [PubMed] [Google Scholar]
- 21.Landrum LM, Java J, Mathews CA, et al. Prognostic factors for stage III epithelial ovarian cancer treated with intraperitoneal chemotherapy: A Gynecologic Oncology Group study. Gynecol Oncol. 2013;130:12–18. doi: 10.1016/j.ygyno.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hodeib M, Eskander RN, Bristow RE. New paradigms in the surgical and adjuvant treatment of ovarian cancer. Minerva Ginecol. 2014;66:179–192. [PubMed] [Google Scholar]
- 23.Chang SJ, Bristow RE. Evolution of surgical treatment paradigms for advanced-stage ovarian cancer: Redefining ‘optimal’ residual disease. Gynecol Oncol. 2012;125:483–492. doi: 10.1016/j.ygyno.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 24.Weeks JC, Uno H, Taback N, et al. Interinstitutional variation in management decisions for treatment of 4 common types of cancer: A multi-institutional cohort study. Ann Intern Med. 2014;161:20–30. doi: 10.7326/M13-2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rogers EM. Diffusion of Innovation. New York, NY: The Free Press; 2003. [Google Scholar]
- 26.Chassin MR. Explaining geographic variations. The enthusiasm hypothesis. Med Care. 1993;31(5, Suppl):YS37–YS44. doi: 10.1097/00005650-199305001-00006. [DOI] [PubMed] [Google Scholar]
- 27.Havrilesky LJ, Alvarez Secord A, Ehrisman JA, et al. Patient preferences in advanced or recurrent ovarian cancer. Cancer. 2014;120:3651–3659. doi: 10.1002/cncr.28940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salani R, Backes FJ, Fung MF, et al. Posttreatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncologists recommendations. Am J Obstet Gynecol. 2011;204:466–478. doi: 10.1016/j.ajog.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Bristow RE, Chang J, Ziogas A, et al. High-volume ovarian cancer care: Survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132:403–410. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 30.Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: Contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11–17. doi: 10.1016/j.ygyno.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]