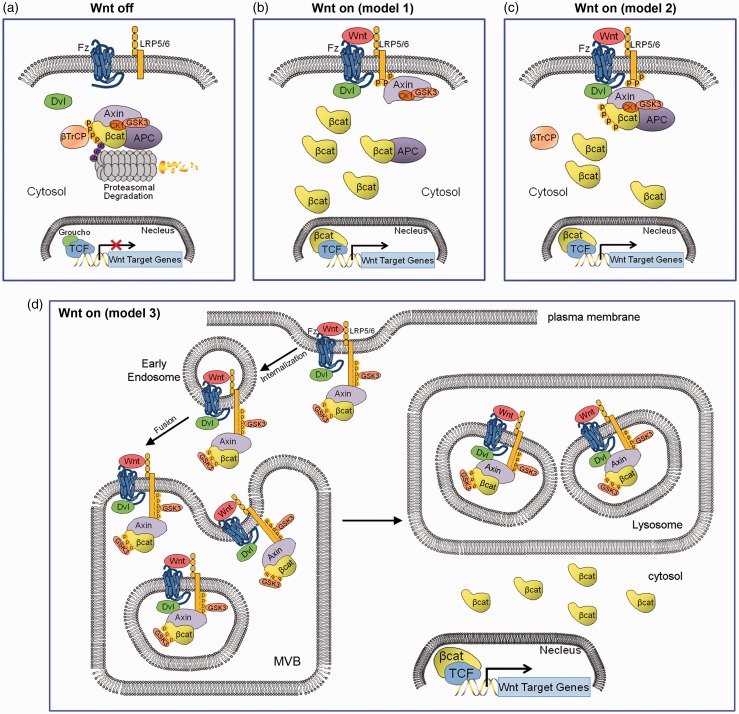
Figure 1.
Models of canonical Wnt signaling. (a) When Wnt receptor complexes are not bound by Wnt ligands on the cell membrane, CK1 and GSK3α/β phosphorylate β-catenin. Phosphorylated β-catenin is recognized by β-TrCP, a component of a dedicated E3 ubiquitin ligase complex. Following ubiquitination, β-catenin is targeted for rapid destruction by the proteasome. In the nucleus, the binding of Groucho to TCF (T cell factor) inhibits the transcription of Wnt target genes. Once bound by Wnt ligand, the Frizzled (Fz)/LRP5/6 coreceptor complex activates the canonical signalling pathway. Fz interacts with Dsh, a cytoplasmic protein that functions upstream of β-catenin. Wnt signaling controls phosphorylation of Dishevelled (Dsh). Wnts are thought to induce the phosphorylation of LRP by GSK3β and casein kinase I-γ (CK1γ), thus regulating the docking of Axin. (b) The recruitment of Axin away from the destruction complex (destruction complex dissociation), (c) or the recruitment of the intact Axin1 destruction complex will lead to the stabilization of β-catenin. In the nucleus, β-catenin displaces Groucho from Tcf/Lef to promote the transcription of Wnt target genes. (d) Canonical Wnt signaling through the sequestration of GSK3 inside multivesicular endosomes. Binding of GSK3 to the Wnt receptor complex, including phospho-LRP6, phospho-β-catenin, and other GSK3 substrates such as Dvl, Axin and APC, sequesters GSK3 inside small intraluminal multivesicular body (MVB) vesicles, causing its cytosolic substrates such as β-Catenin (in yellow) and many other Wnt/STOP target proteins to become stabilized. The initial GSK3 molecules are recruited to the receptor complex bound to Axin, ensuring that the GSK3 fraction bound to the destruction complex is first being depleted. (A color version of this figure is available in the online journal.)