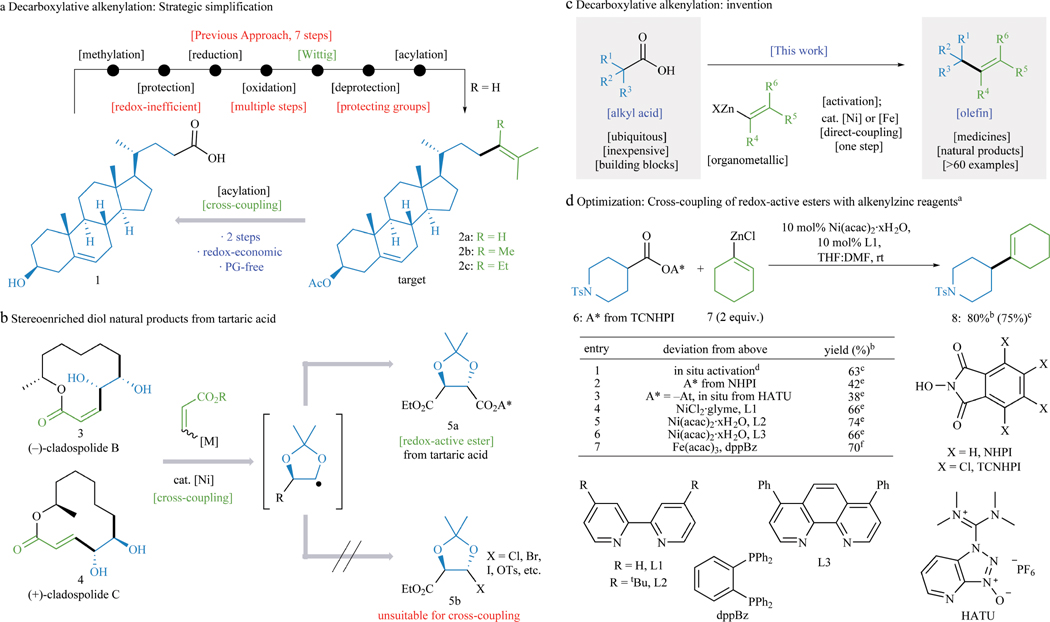
Figure 1. Development of Ni– and Fe–catalyzed decarboxylative alkenylation.
a, Conventional route to sterol acetates (2a–c). b, Utilization of previously unavailable electrophiles in cross-coupling reactions. c, Decarboxylative alkenylation presents a potential solution. d, Optimization of decarboxylative alkenylation. a0.1 mmol. bYield by 1H NMR with CH2Br2 internal standard. c0.25 mmol scale, isolated. d1.1 equiv. TCNHPI, 1.1 equiv. DIC, CH2Cl2 (0.2 M). e20 mol% [Ni] and L, 3.0 equiv. alkenylzinc. f10 mol% [Fe], 12 mol% dppbz, 1.5 equiv. dialkenylzinc. See Supporting Information for additional details.