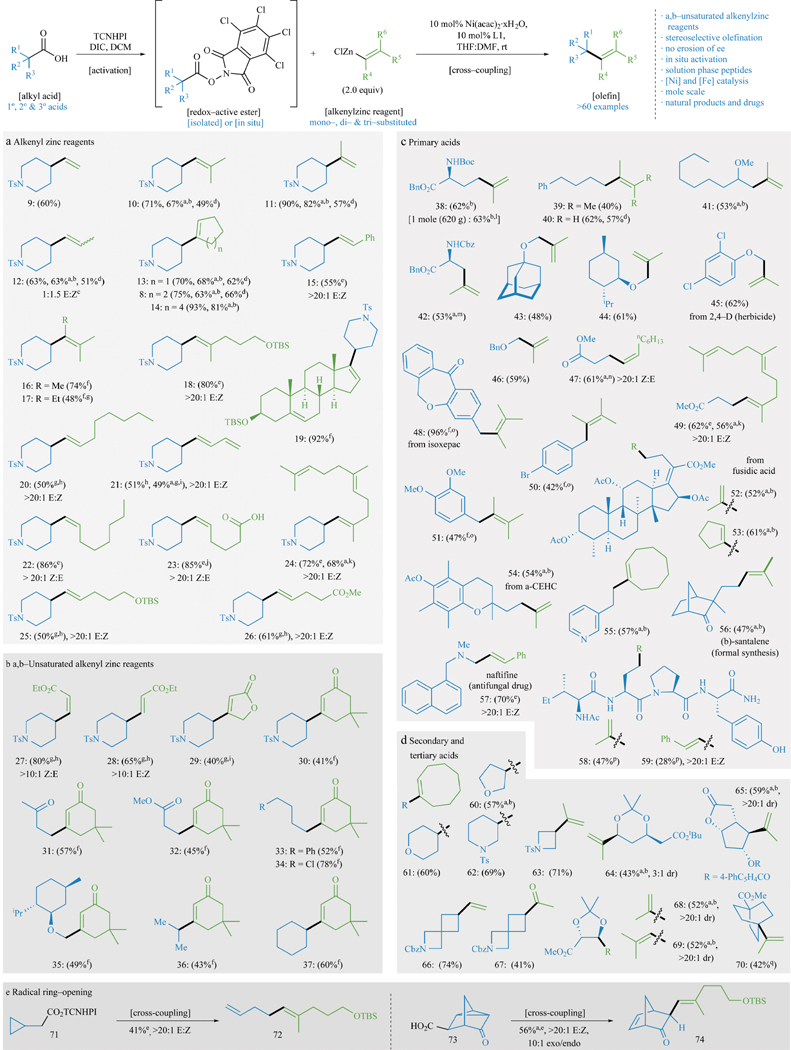
Figure 2. Substrate scope of decarboxylative alkenylation.
The carboxylic acid component is shown in blue and the alkenyl zinc reagent is shown in green. Cross-coupling using various alkenyl zinc reagents (a, b) and different primary (c), secondary and tertiary (d) acids evaluated. Yields refer to isolated yields of products after chromatography on SiO2. a in situ activation. b 3 equiv. alkenylzinc. c alkenylzinc derived from commercial Grignard that exists as a mixture of olefin isomers. d 10 mol% Fe(acac)3, 12 mol% dppbz, 1.5 equiv. dialkenylzinc. e 2 equiv. alkenylzinc, 2 equiv. MgBr2·OEt2. f MeCN as solvent. g 60 °C. h 20 mol% Ni/L, 3 equiv. alkenylzinc, 3 equiv. MgBr2·OEt2. i 20 mol% Ni/L, 5 equiv. alkenylzinc, 5 equiv. MgBr2·OEt2. j alkenylzinc derived from OBO-ester; see SI for work up details. k 3 equiv. alkenylzinc, 3 equiv. MgBr2·OEt2. l See SI for details regarding mol-scale reaction. m 4 equiv. alkenylzinc. n 4 equiv. alkenylzinc, 4 equiv. MgBr2·OEt2. o 10 mol% NiCl2·glyme/4,4′-di-tBuBipy. p See SI for details regarding peptide substrates. q 20 mol% Ni/L, 5 equiv. alkenylzinc, NMP.