Abstract
CD147 is highly expressed on the surface of numerous tumor cells to promote invasion and metastasis. Targeting these cells with CD147-specific antibodies has been validated as an effective approach for lung and liver cancer therapy. In the immune system, CD147 is recognized as a co-stimulatory receptor and impacts the outcome of thymic selection. Using T cell-specific deletion, we showed here that in thymus CD147 is indispensable for the stable αβ T cell lineage commitment: loss of CD147 biases both multipotent DN (double negative) and fully committed DP (double positive) cells into innate NK-like lineages. Mechanistically, CD147 deficiency results in impaired Wnt signaling and expression of BCL11b, a master transcription factor in determining T cell identity. In addition, functional blocking of CD147 by antibody phenocopies genetic deletion to enrich NK-like cells in the periphery. Furthermore, using a melanoma model and orthotopic liver cancer transplants, we showed that the augmentation of NK-like cells strongly associates with resistance against tumor growth upon CD147 suppression. Therefore, besides its original function in tumorigenesis, CD147 is also an effective surface target for immune modulation in tumor therapy.
Keywords: BCL11b, Cancer immune-therapy, CD147, Trans-differentiation, Thymocyte development
Highlights
-
•
DN, DP cells were reprogrammed into innate NK-like cells after thymic CD147 deleted
-
•
Loss of CD147 results in impaired Bcl11b expression and T-lineages development, which can be rescued by Wnt3a stimulation.
-
•
CD147 is an vital target for immune modulation via NK-like cells in tumor therapy.
Tumor therapy is a difficult task and many methods have been used. Among them, tumor immunotherapy is a focus in the field and has made great progress. In this study, we found CD147 is an vital target for immune modulation via NK-like cells in tumor therapy, which means CD147 antibody may be through regulating immune cells to achieve tumor therapy. Although CD147 antibody has been used for liver cancer, making clear the mechanism of CD147 antibody mediated tumor therapy may be benefit for guiding clinical treatment.
1. Introduction
T cell development is a process that restricts lineage choices of common lymphoid precursors (CLPs) and lymphoid-primed multipotent precursors (LMPPs) in a step-by-step fashion. The thymus is a permissive microenvironment for T-cell lineages by providing spatiotemporally structured signals to instruct CLPs and LMPPs to differentiate in a fixed direction (Ciofani and Zuniga-Pflucker, 2007). These signals drive lymphoid precursors to become early thymic progenitors (ETP) cells, to become CD4/CD8 double negative (DN, DN2-DN3-DN4), to become CD4/CD8 double positive (DP), then finally to become either CD4 or CD8 single positive thymocytes (SP). Recent progress has unveiled that thymic T-lineage commitment and maintenance requires activation of several key transcription factors (Yui and Rothenberg, 2014). Among them, Bcl11b plays a dominant role in each step of thymocyte development. During phase I of the lineage choice decision (ETP to DN2a) (Yui and Rothenberg, 2014), the loss of Bcl11b results in the continuous expression of genes that maintains stemness and multipotency and differentiation of early thymocytes into the myeloid and NK lineages (Ikawa et al., 2010, Li et al., 2010a, Li et al., 2010b). In addition, Bcl11b-deficient cells that have passed through the first stage of lineage choice and have committed to the T cell lineage are still susceptible to reprogram into an NK-like cell lineage(Kastner et al., 2010, Li et al., 2010b). While the lineage-specifying transcription factors for T cells have been extensively studied, upstream mechanisms have remained to be investigated. Specifically, it is intriguing to know how these transcription factors are regulated by external signals, and wha surface receptors are responsible for lineage stability in committed T cells.
CD147, also known as Basigin or EMMPRIN, is a highly glycosylated immunoglobulin superfamily protein. Under physiological conditions, this transmembrane protein is widely expressed and plays fundamental roles in various hematopoietic and non-hematopoietic cell lineages (Fok et al., 2014, Hahn et al., 2015, Kaushik et al., 2015, Pennings and Kritharides, 2014). In various cancers, levels of CD147 expression are highly elevated and are associated with poor prognosis (Als et al., 2007, Caudroy et al., 1999, Sienel et al., 2008, Xiong et al., 2014). Beyond its role as a tumor biomarker, CD147 is considered as a bona-fide tumor associated antigen (TAA) because of its intrinsic regulation in tumorigenesis (Biswas et al., 1995, Sweeny et al., 2013, Tang et al., 2005, Wang et al., 2015). Besides its critical role in tumorigenesis, CD147 is also identified as a signal receiver for immune modulation, especially in T cell development and activation. Throughout the entire T cell lineage, from ETPs to naïve T cells and to fully differentiated memory cells, CD147 is highly expressed (Kirsch et al., 1997, Renno et al., 2002). Blocking CD147 with a specific monoclonal antibody partially arrested thymocyte development at both the DN3 to DN4 transition and DP to CD4+ SP selection (Renno et al., 2002), and the latter phenotype was validated by Lck-Cre driven CD147 genetic deletion (Yao et al., 2013). These developmental abnormalities could indicate that loss of CD147 impairs pre-TCR/TCR signaling; but it may alternatively suggest that CD147 functions in other critical pathways that facilitate thymocyte development besides TCR signaling.
In this study, we analyzed thymocyte development in a mouse strain that carries the conditional CD147 allele and transgenic Cre recombinase under the control of the proximal Lck promoter (CD147T-KO mice (Yao et al., 2013)). Using this model, we made a surprising discovery that the loss of thymic cellularity and diminished cells of the T-cell lineage was accompanied by an increase of various lymphocyte populations with innate immunological functions, such as γδ T cells, NKT-like cells, and NK-like cells. We further assessed the lineage conversion capacity of these cells, dissected their associated molecular mechanism, and determined that CD147 could serve as an immune modulatory target for antibody-mediated cancer therapy.
2. Materials and Methods
2.1. Mice
CD147T-KO conditionally knocked out mice were developed according to a standard gene targeting approach in ES cells, and were used in previous experiments (Kuno et al., 1998). CD147T-KO mice were of a mixed C57BL/6 J and 129S5 genetic background. Normal female C57BL/6 mice were purchased from the Fourth Military Medical University, Laboratory Animal Center, and white C57BL/6 mice were obtained from the Biomedical Analysis Center of the Third Military Medical University. The detail information can be found in Supplemental materials and methods.
2.2. Reprogramming of T cells to NK-like Cells In Vitro
Myeloid cells were obtained from mouse bone marrow after injection of 5-FU for 4 days, and DP cells were sorted by FACS. 105 cells/well of myeloid cells or DP cells were cultured on OP9-DL1 stromal cells in media (alpha-MDM with 20% FCS, 1% penicillin/streptomycin, and 2 mM l-glutamine, 5 × 10–5 M β-mercaptoethanol, 10 mM Hepes buffer, sodium pyruvate, 5 ng/ml IL-7 (R&D), and 27.5 ng/ml Flk2/Flt3 (R&D)). Every 3 days, half of the media was replaced with new media for 7 to 21 days. DN3 or DP thymocytes were sorted by FACS, and likewise co-cultured with OP9-DL1 in T cell culture media (RPMI-1640, 10% FCS, 1% penicillin/streptomycin, 2 mM l-glutamine, 5 ng/ml IL-7, 27.5 ng/ml Flk2/Flt3) at 3000 cells per well in 24-well plates. 100 ng/ml huIL-2 was supplemented in T cell medium to promote NK-like cell proliferation. Every 3 days, half of the media was replaced with fresh T cell media with IL-2.Every seven days, cells were collected by vigorous pipetting, filtered through cell strainers and transferred to new tissue culture plates pre-seeded with fresh OP9-DL1 stromal cells. Cells were collected and analyzed by FACS after 14 days.
2.3. Reprogramming of Single Thymocyte to NK-like Cells
Single DN3 thymocyte was sorted directly into individual wells in a 96-well plate pre-seeded with OP9-DL1 stromal cells in T cell medium supplemented with 100 ng/ml huIL-2.Medium was changed every 3 days. After 12 days cells were analyzed by flow cytometry.
2.4. Cytotoxicity Assays for Converted NK-like Cells
Target cells, B16-F10 melanoma cells, were maintained in RPMI-1640,10% FCS, 1% penicillin/streptomycin, 2 mM l-glutamine. NK-like cells were generated in vitro with OP9-DL1 feeder cells as described above. To perform the cytotoxicity assay, target cells were washed and incubated with 0.1 μCi Na251CrO4 for 45 mins at 37 °C. 51Cr-loaded cells were then washed and mixed with to-be-tested effector cells at various ratios, and then incubated for 4 h at 37 °C before the supernatant was tested for chromium release in a scintillation counter. Percent specific lysis was calculated as (experimental release − spontaneous release) / (maximum release − spontaneous release) × 100.
2.5. Retroviral Transduction of Mouse Bone Marrow Cells
The day before transduction, PLAT-E packaging cells were plated at 1 × 106cells/well of a 6-well plate in DMEM with 10% FCS. After 24 h, the cells were transfected with MSCV-Puro-2Xins-mG-Mock vectors carrying TCF1 and Neo cDNAs using Fugene 6 transfection reagent (Roche) according to the manufacturer's instructions. 24 h after transfection, medium was replaced and the plate was transferred to 32 °C for retrovirus production. The viruses were collected at 48 h and 72 h, and filtered with a 0.45 μm filter before transduction. Twenty-four hours after transduction, the medium was replaced. Mouse bone marrow cells were seeded at 8 × 105 cells per 100 mm dish. After 24 h, virus-containing supernatants derived from these Plat-E cultures were filtered through a 0.45 μm cellulose acetate filter (Schleicher & Schuell) and supplemented with 4 μg/ml polybrene (Nacalai Tesque). Target cells were incubated in the viral/polybrene-containing supernatant for a minimum of 4 h. After infection, the cells were replated in 10 ml fresh medium. 3 days after infection, G418 was added at a final concentration of 0.3 mg/ml, and the GFP+ cells were sorted by FACS Aria.
2.6. In Vivo Tumor Transplantation
6 week old female C57BL/6 mice were used in all experiments. A murine in situ hepatoma model was generated by intraperitoneally anesthetizing mice with 50 mg/kg of pentobarbital. The mice were fixed, and their abdomens dissected to expose their liver. 1 × 106 viable Hepa16 cells or Hepa16-IRES cells in 0.05 ml DMEM were intrahepatically injected into murine liver. 10 mg/kg of CD147 antibody (R&D, Clone # 116318) was used for treatment starting on day 3, and treatment was given every three days for two weeks. Small animal imaging was performed on hepatoma-bearing mice on day 3, 7, 14, 28 and 42, and the livers were removed at day 3, 7 and 14, and were weighed to determine the tumor growth. The number of NK cells were quantified using flow cytometry and immunofluorescence. Black C57BL/6 mice were used in melanoma model. 5 × 104 viable B16-F10 cells resuspended in 0.02 mL DMEM were subcutaneously injected, and tumor size was detected starting on day 6. 10 mg/kg of CD147 antibody treatment was carried out from day 1, and treatment was given every three days for four times. The number of NK cells were again quantitated using flow cytometry and immunofluorescence.
2.7. Statistical Analysis
Graphpad Prism software was used to analyze the data. Means, S.D. and the probability (p) were presented in some figures. ANOVA were used to compare when divided into more than two groups, and student's t-test was used to assess the comparisons between two groups. Comparisons of tumor-growth curves were assessed by analysis of variance. *p < 0.05 was considered significant.
The other methods can be found in Supplemental materials and methods.
3. Results
3.1. Loss of CD147 Biases Developing T cells Into NKT-like Lineages
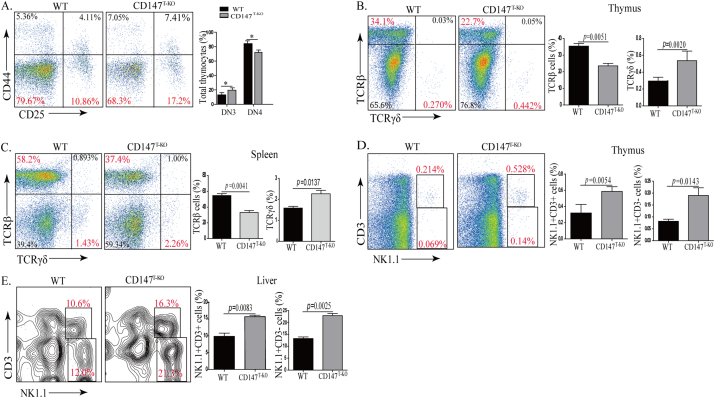
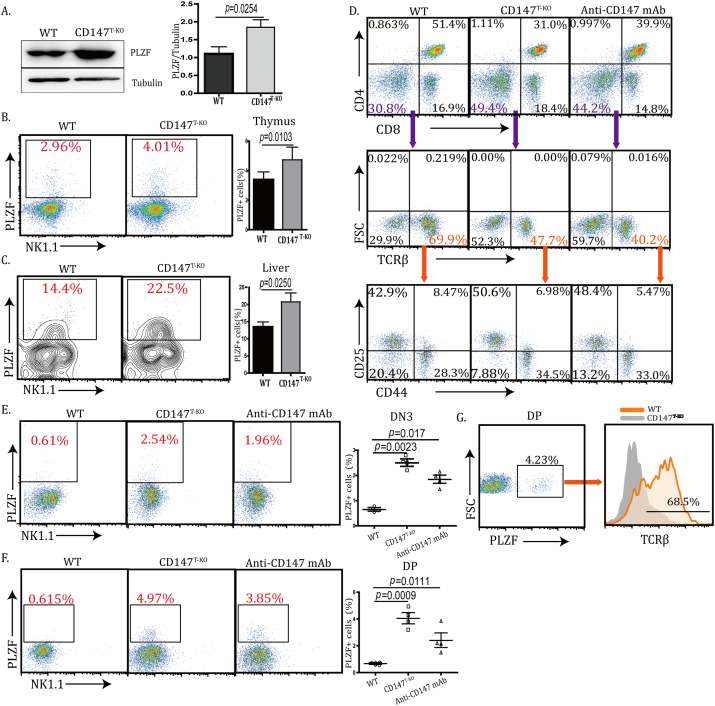
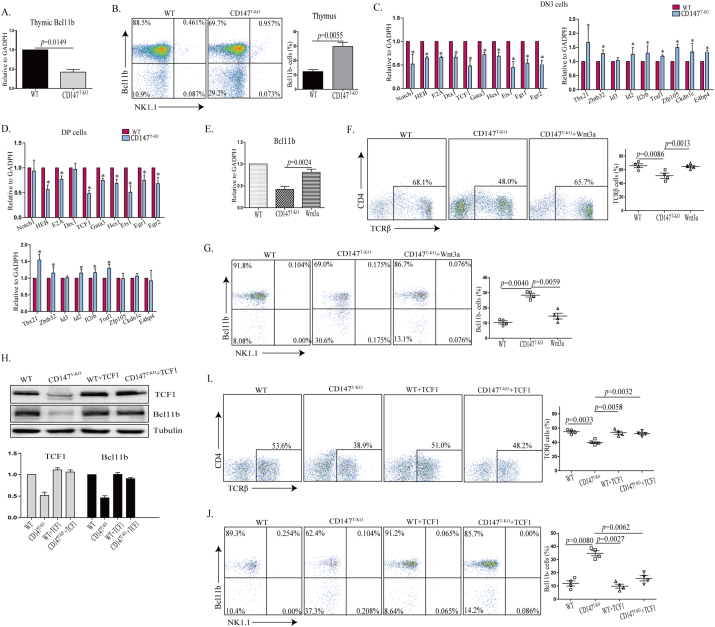
In fetal thymic organ culture, suppression of CD147 resulted in a transitional block of developing thymocytes from the DN to DP stage. In another report, this blockade was established as early as the DN1 stage (Renno et al., 2002). Through the proximal Lck promoter-driven Cre expression, we previously reported that the loss of CD147 dampened thymic cellularity in various populations (Yao et al., 2013). We revisited these CD147T-KO mice to assess detailed roles of CD147 during thymic development. Using the thymus from 6-week-old mice, we detected again a significant reduction in thymic weight in the CD147T-KO mice compared to those in the control mice (Fig. S1A). In addition, the total number of thymocytes in the CD147T-KO mice was significantly lower than that of the control mice (Table. S1). Besides the reduction of total cellularity, we observed that the percentage of total DN cells was increased in the CD147T-KO thymus, accompanied with disproportionate decrease of the DP population (Fig. S1B & Table S2). We examined whether this decrease in cell number was due to the excessive amount of cell death in the DP cell population in the absence of CD147 signaling. Using freshly isolated thymocytes, we performed FACS-aided TUNEL staining, and found no difference in the ratios of apoptotic DP thymocytes between wild type (WT) and CD147T-KO mice (Fig. S1C). We reasoned that the contraction of the DP population was most likely due to impaired transition from the DN to DP stage. In addition, we observed a reproducible percentage increase of DN3 cells and relative contraction of the DN4 population, indicating a DN3 to DN4 block (Fig. 1A). Taken together, the above results validate that CD147 is indispensable for immature thymocyte development. We examined CD147T-KO lymphocytes in peripheral blood, lymph nodes, and spleens. Unlike the observed defects in thymus, in comparison to their littermate controls, the total number of splenocytes was similar in CD147T-KO mice (Fig. S2A & Table S3). Furthermore, ratios are normal between T and B cells; and the CD4+ to CD8+ ratios were also comparable to that of WT mice (Fig. S2B–C & Table S4). Similar results were found in the lymph node and blood (Fig. S2D). Given this, we speculated that the moderate developmental defect in the thymus might be compensated by peripheral homeostatic expansion after CD4+ and CD8+ SP thymocytes egress. We further characterized other innate-like lymphocyte populations in the CD147T-KO mice. Accompanying the loss of αβ T cells, the percentage of γδ T cells increased 1.83-fold (Fig. 1B) in the thymus of CD147T-KO mice and 1.44-fold in spleen (Fig. 1C); the percentage of thymic ΝΚΤ and NK-like cells increased 2.47-fold and 2.25-fold, respectively (Fig. 1D). Since the liver is a peripheral organ in which NK and NKT cells are enriched (Gao et al., 2009), we analyzed these populations and found that, within hepatic leukocytes, the percentage of NKT and NK-like cells increases from 9.45 ± 3.52%(WT) to 15.43 ± 3.89% (CD147T-KO) and 11.62 ± 3.94% (WT) to 20.54 ± 4.12% (CD147T-KO), respectively (Fig. 1E). Accordingly, absolute numbers of NK1.1+ cells in thymi and livers were also expanded in the CD147T-KO mice compared to those in the control mice (Table S5), which indicated that the expansion of NKT- and NK-like populations was an absolute increase rather than a relative enrichment. This bias towards non-αβ T cells – the enrichment of γδ T cells and NK-lineage cells — was also evident in other peripheral lymph organs, such as the lymph node, spleen and blood (Fig. S3A–C). To validate the NK-like lineage bias indicated by cell surface markers, we analyzed the thymic expression of multiple lineage-specific regulators on CD147 deletion. PLZF is a transcription factor that is both necessary and sufficient to enforce the innate effector program of NKT cells (Constantinides and Bendelac, 2013). We identified that PLZF protein levels were increased in CD147T-KO thymus (Fig. 2A), which is largely due to the increase of thymic PLZF+ populations (Fig. 2B). A similar proportional increase of PLZF+ cells was also identified in the livers of CD147T-KO mice (Fig. 2C). To determine the source of this increase, we sorted Lin-Sca1+ c-Kit+ hematopoietic stem cells (HSCs) from the bone marrow of WT and CD147T-KO mice and cultured them side-by-side on OP9-DL1 stromal feeder cells under the standard T-lineage induction conditions (Schmitt and Zuniga-Pflucker, 2002). By day 7, 71.4 ± 6.52% of WT HSCs differentiated into CD4− CD8− TCRβ+ cells, but this differentiation was significantly inhibited (50.65 ± 5.74% CD4-CD8-TCRβ+) upon CD147 deletion initiated at the late DN2 stage. Within the TCRβ+ population, the ratio between the DN3 and DN4 cells changed from 2:1 to 6:1, which recapitulates the in vivo developmental phenotype of CD147T-KO mice (Fig. 2D). This inhibition of T cell development was accompanied by a significant increase of PLZF+ cells in the CD147T-KO HSC culture. This biased differentiation can be reproduced using WT HSCs when a functional blocking antibody against CD147 was applied to the culture (Fig. 2D, E). A similar biased development of PLZF+ cells was seen when sorted CD147T-KO DP thymocytes were applied to an OP9-DL1-supported culture (Fig. 2F), and about 70% of these PLZF+ cells were expressed TCRβ (Fig. 2G). Taken together, these data show that PLZF+ NKT-like cells preferentially develop at multiple stages of T cell development upon CD147 deletion or functional suppression.
Fig. 1.
CD147 deletion in T cells lead to an increase in innate-like lymphocytes. A. Analysis of DN1-DN4 thymocytes from WT and CD147T-KO mice using flow cytometry. *p < 0.05; **p < 0.01; NS, no significance. B—C. Analysis of TCRβ T cells and TCRγδ T cells in thymus (B) and in spleen (C) of WT and CD147T-KO mice using flow cytometry. D–E. Analysis of NK and T cells in thymus (D) and in liver (E) of WT and CD147T-KO mice using flow cytometry. Data are representative 6 (A) or 4 (B–E) experiments.
Fig. 2.
CD147 deletion in T cells lead to an increase of PLZF+ NKT-like cells. A. Analysis of PLZF protein expression in the thymus of WT and CD147T-KO mice by western blot. B—C. Analysis of PLZF+ cells in the thymus (B) and in liver (C) of WT and CD147T-KO mice using flow cytometry. D. Bone marrow hematopoietic stem cells were co-cultured on OP9-DL1 cells without IL-2, and then collected after 7 days. The CD4 vs. CD8, TCRβ and CD25 vs. CD44 populations were detected by flow cytometry. E. Bone marrow hematopoietic stem cells were enriched and co-cultured on OP9-DL1 cells without IL-2, and then collected after 14 days. PLZF+ cells were analyzed using flow cytometry. F. DP thymocytes were co-cultured on OP9-DL1 cells without IL-2, and then collected after 21 days. PLZF+ cells were analyzed by flow cytometry. G. Analysis of TCRβ expression in PLZF+ cells after DP thymocytes were co-cultured on OP9-DL1 cells for 21 days without IL-2. Data are representative 4 (A–C) or 3 (D–G) experiments.
3.2. Loss of CD147 Reprograms Committed T cells Into NK-like Lineage
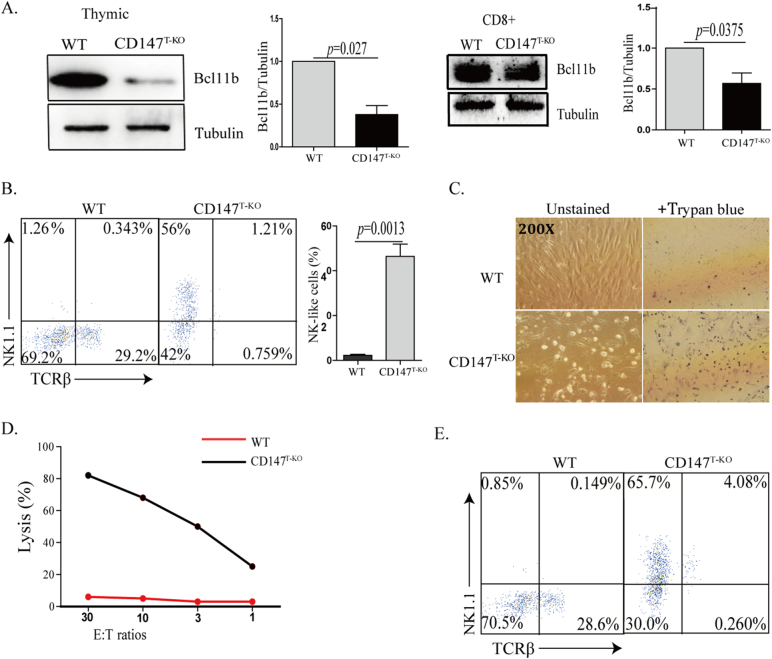
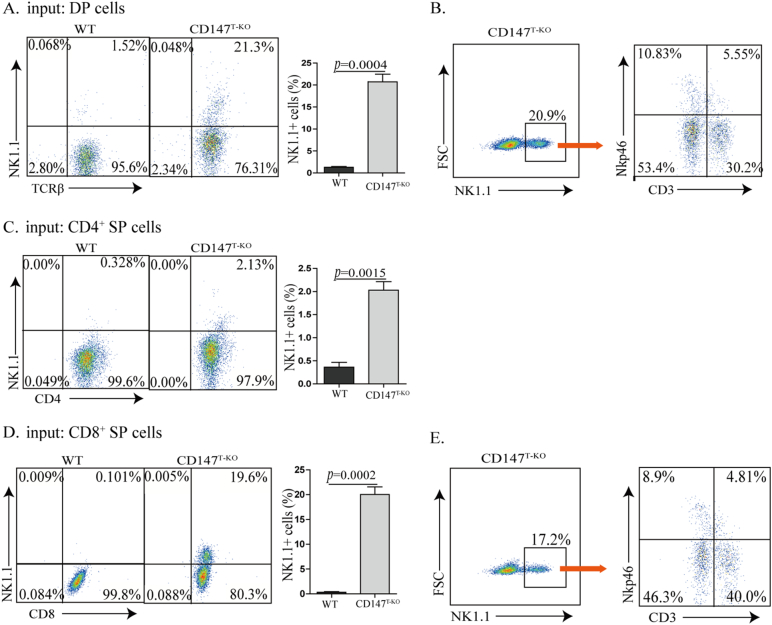
It has been reported that genetic deletion of Bcl11b reprograms multiple stages of lineage-committed T cells into NK-like cells (Li et al., 2010b). Unlike the development of NKT cells, this in vitro NK-like reprograming is usually associated with the downregulation of TCRβ (Li et al., 2010b), which we observed in our OP9-DL1 culture using sorted CD147T-KO HSCs (Fig. 2D). In addition, when CD147T-KO thymocytes and CD8+ T cells were subjected to western blot analysis, we found expression of Bcl11b was significantly reduced (Fig. 3A). To examine whether this is due to a T to NK cell conversion, sorted DN3 thymocytes from CD147T-KO and WT mice were cultured on OP9-DL1 stromal feeder cells for 14 days, supplemented with 100 ng/ml of IL-2. As Liu and colleagues similarly observed by using an inducible Bcl11b gene deletion (Li et al., 2010b), we found that loss of CD147 resulted in more than a 30-fold increase of NK-like cells and more than a 30-fold reduction of T cells (Fig. 3B). Furthermore, these DN3 T cells-converted NK cells are highly cytotoxic, as a majority of OP9-DL1 cells were killed during this trans-differentiation process (Fig. 3C) and > 80% of these cells expressed granzyme B (Fig. S4A). While these day 14 culture products were subjected to standard 51Cr-release assays, NK-like cells converted from CD147T-KO DN3 thymocytes were fully capable of producing cytotoxicity to kill B16-F10 melanoma cells (Fig. 3D). To fully assess the capacity of this conversion, we sorted single WT or CD147T-KO DN3 cells into individual culture wells supplied with OP9-DL1 feeder cells and IL-2. After 12 days of culture, while < 4% (2/51) of WT culture wells have detectably NK cells, reprograming was detected in 66% (35/53) of CD147T-KO wells. Within these wells, around 65% of the highly expanded cells were NK1.1+ (Fig. 3E), indicating that NK cells can efficiently be generated from CD147 deficient DN3 cells in an optimal environment. We further subjected more matured CD147T-KO T cells (DP, CD4+ SP and CD8+ SP) to this in vitro trans-differentiation test. Similar to DN3 cells, after 14 days of culture, all T lineages were partially converted into NK1.1+ cells and expanded in the presence of IL-2 (Fig. 4). Among them, CD4+ CD147T-KO cells were relatively more stable than other two populations (Fig. 4C vs A, D). Within the converted NK1.1+ cells we detected a significant portion of them are CD3-NK1.1+ Nkp46+, which phenotypically indicated complete trans-differentiation (Fig. 4B, E). Importantly, the converted cells are highly cytotoxic (Fig. S4B). To evaluate the conversion of peripheral T cells, particularly CD8+ T cells, we sorted CD8+ T cells from the spleen. Supplemented with 100 ng/ml IL-2, the cells were co-cultured with OP9-DL1 feeder cells for 14 days. The results showed that a portion of CD147T-KO peripheral CD8+ T cells acquired NK1.1 (Fig. S4C), which indicated CD147T-KO peripheral CD8+ T cells have the potential to be converted into NK-like cells (Fig. S4C), although we cannot exclude the possibility that the conversion process of these peripheral T cells are initiated in the thymus. Nevertheless, these results mirrored the in vitro conversion phenotypes of cultured Bcl11b-depleted cells (Li et al., 2010b). In addition, we also analyzed expression of NK1.1 in thymic and peripheral CD8+ T cells. In all examined peripheral organs, we observed the increase of CD8+ NK1.1+ cells in CD147T-KO mice (Fig. S4D). Taken together, similar to a genetic Bcl11b deletion, the loss of CD147 also weakens the stability of lineage commitment of various T cells, but instead strengthens their potential to differentiate into NK-like cells.
Fig. 3.
CD147 deletion reprograms DN3 cells into the NK-like lineage. A. Analysis of thymic and peripheral CD8+ Bcl11b expression by western blot in both WT and CD147T-KO mice. B. Flow cytometry analysis of DN3 thymocytes co-cultured with OP9-DL1 feeder cells for 14 days, supplemented with 100 ng/ml IL-2. C. Trypan blue staining was used to detect the death of OP9-DLI stromal cells induced by killing from co-cultured DN3 thymocytes supplemented with 100 ng/ml IL-2. Images were captured at 200 × magnification. D. Cytotoxicity of DN3-converted cells measured in standard 51Cr-release assays with B16F10 tumor cell targets at the indicated effector-to-target (E:T) ratios. E. Flow cytometry analysis of surface makers of culture products that were reprogrammed from single CD147T-KO DN3 cells supplemented with 100 ng/ml IL-2. Data are representative of 4 (A) or 3 (B, E) experiments.
Fig. 4.
CD147 deletion reprograms DP, CD4, and CD8 T cells into an NK1.1+ cell lineage. DP, CD4SP, CD8SP T cells were co-cultured with OP9-DL1 feeder cells for 14 days in the presence of 100 ng/ml IL-2. A. Flow cytometry analysis of co-cultured DP thymocytes with NK1.1 and TCRβ. B. CD3-NK1.1+ Nkp46+ cells were detected in CD147T-KO DP-converted cells. C. Flow cytometry analysis of co-cultured CD4 T cells with NK1.1 and TCRβ. D. Flow cytometry analysis of co-cultured CD8 T cells with NK1.1 and TCRβ. E. CD3-NK1.1+ Nkp46+ cells were detected in CD147T-KO CD8+-converted cells. Data are representative of 3 experiments.
3.3. CD147 Facilitates Wnt Signaling and Bcl11b Expression to Maintain Lineage Specificity of T cells
Since Bcl11b is haplosufficient in supporting a normal T cell development program (Li et al., 2010b), the partial reduction of Bcl11b transcripts detected in CD147T-KO thymocytes predicts that, while the others maintain normal Bcl11b expression, a sub-group of CD147T-KO thymocytes completely lost this protein (Fig. 5A). Intracellular staining of Bcl11b in a mixed population of thymocytes showed a 3-fold increase of Bcl11b negative cells in the thymus of CD147T-KO mice, while its levels of expression remain comparable in the Bcl11b+ subgroup (Fig. 5B). This result suggests that deleting CD147 as early as the late DN2 stage can stochastically silence Bcl11b gene expression in maturing T cells.
Fig. 5.
CD147 is indispensable to support optimal Wnt signaling and Bcl11b expression. A. qRT-PCR validation of thymic Bcl11b expression. B. Analysis of Bcl11b expression in the thymus using flow cytometry. C. T-cell development related genes (Notch1, HEB, Hes1, Dtx1, TCF1, Gata3, Ets1) and NK or NKT or NK-like cells related genes (Tbx21, Id3, Id2, Zfp105, Il2rb, E4bp4, Zbtb32, Traf1, Ckdn1c) were selected for qRT-PCR analysis. qRT-PCR validation of the gene expression of selected genes from sorted DN3 cells. D. qRT-PCR validation of the gene expression of selected genes from sorted DP cells. E–G. Bone marrow hematopoietic stem cells were co-cultured with OP9-DL1 cells. Wnt3a was then added from begining, and cells were collected for total RNA after 7 days. qRT-PCR was used to analyze Bcl11b expression (E) and flow cytometry analysis of TCRβ cells (F) and flow cytometry analysis of Bcl11b expression (G). H. TCF1 cDNA was transduced into CD147T-KO cells from mouse bone marrow with MSCV-Puro-2Xins-mG-Mock retroviral vector. Western blot analysis of TCF1 and Bcl11b expression was performed from sorted TCF1 transduced cells. I–J. Bone marrow hematopoietic stem cells that were transfected with TCF1 were co-cultured with OP9-DL1 cells, and then collected after 7 days. Flow cytometry analysis was performed of TCRβ cells (I) and Bcl11b expression (J). Data are representative of 4 experiments.
To determine the molecular mechanisms for CD147 maintenance of T cell lineage specificity with sorted WT and CD147T-KO DN3 thymocytes, we examined the expression levels of signature genes for T and NK-like lineages ex vivo. Quantitative PCR analysis showed a general upregulation of a series of genes controlling NKT/NK development and effector function, such as T-bet (Tbx21), Id2, IL2Rb, and ZBTB32. On the contrary, expression of genes involved in T cell lineage formation, such as molecules related to Notch signaling (Notch1, Hes1 and Dtx1) and crucial transcription factors for lineage specification such as Ets1, GATA3 and TCF1 were suppressed in CD147T-KO thymocytes (Fig. 5C). A similar gene expression profile, was observed when DP cells were examined (Fig. 5D); however, there was no significant difference in genes related to Notch signaling, which correlates with a reduced Notch dependency in the DP population. Although a Notch signaling defect was observed in CD147T-KO thymocytes, and CD147 was proposed to be a subunit of γ-secretase in Hela cells, functional examination indicated it is unlikely that CD147 participates directly in Notch pathway (Vetrivel et al., 2008, Zhou et al., 2005).
In various lung carcinoma cell lines, overexpression of CD147 facilitates β-catenin nuclear translocation and enhances Wnt signaling (Sidhu et al., 2010). As a key mediator of canonical Wnt signaling effect in early thymocyte development (Staal et al., 2008), transcription factor TCF-1 plays an essential role in early T-lineage specification (Verbeek et al., 1995, Weber et al., 2011). Specifically, ectopic expression of TCF-1 in lymphoid-primed multipotent progenitors elicits Notch-independent T-lineage differentiation and the expression of Bcl11b (Weber et al., 2011). Given this, we investigated the causal link between CD147, Wnt signaling, and Bcl11b expression. In the OP9-DL1 supported CD147T-KO HSC cultures, treatment with a high dose of Wnt3a significantly elevated Bcl11b expression as compared to CD147T-KO cells (Fig. 5E, G), and fully restored the impaired T-lineage differentiation (Fig. 5F). In addition, on the CD147T-KO genetic background, overexpression of TCF-1 elevated Bcl11b expression in developing T cells (Fig. 5H). For individual cells, this elevation fully restored lost Bcl11b expression (Fig. 5J) and directly rescued the defect of T-lineage commitment (Fig. 5I). Taken together, these rescue results indicate that CD147 is indispensable to support optimal Wnt signaling, which was unexpectedly required for optimal Notch activation and consequent Bcl11b expression.
3.4. CD147 as an Immunomodulation Target for Tumor Therapy
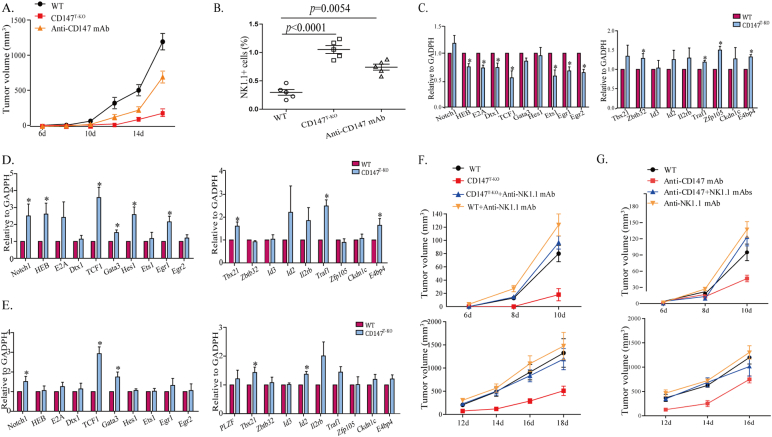
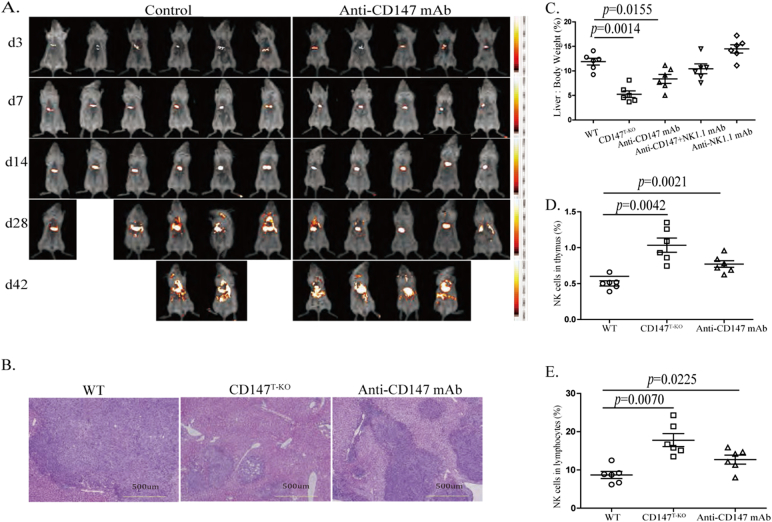
It has been demonstrated that NK-like cells that have converted from Bcl11b-deficient DP thymocytes have superior cytotoxicity against B16 melanoma cells in vitro and in vivo (Li et al., 2010b). Clinically, anti-CD147 antibody alone has proven to suppress primary tumor growth and hepatocellular carcinoma metastasis (Wang et al., 2015, Zhang et al., 2015). While these anti-tumor efficacies were largely attribute to tumor-specific targeting and blocking of CD147 function in tumor cells, we hypothesized that CD147 may also serve as a modulatory target to reprogram T cells into superior NK-like cytotoxic cells, which may be a complimentary mechanism for the therapeutic effect of anti-CD147 blocking. To examine this hypothesis, we first transplanted recipient mice subcutaneously with B16 melanoma cells and found that cells had high surface CD147 levels, but low MHC-I expression (Fig. S5A, B). Compared to WT group, deletion of CD147 in the T cell lineage resulted in a significant retardation of melanoma progression (Fig. 6A). This enhanced surveillance against the tumor correlates with an intratumoral enrichment of NK1.1+ cells in both the TCR+ and TCR− populations (Fig. 6B, S5C & S5D). In addition, treating tumor-bearing mice with an anti-CD147 monoclonal antibody also effectively reduced tumor burdens in transplanted WT mice (Fig. 6A), and was also associated with enhanced infiltration of NK-like cells into tumor beds (Figs. 6B & S5C). To determine the molecular characteristics of these NK-like cells, we FACS sorted CD3+ CD8+, CD3− NK1.1+, and CD3+ NK1.1+ cells from day14 B16 melanoma and performed quantitative PCR analysis with a panel of characteristic genes in T and NK lineages. We observed that, in comparison to WT, 1) for CD147-deficient tumor infiltrating CD8+ T cells, although it was moderate, T cell lineage characteristic genes were generally suppressed; the expression of NK-lineage characteristic genes were increased (Fig. 6C). This indicated that the developmental bias identified in tumor-free animals can also be identified in tumor infiltrating T cells; 2) probably more importantly, for CD147-deficient tumor infiltrating NK and NKT cells, we observed that while expression levels of NK-lineage biased genes were comparable to WT NK or NKT cells, expression levels of T-lineage genes were significantly higher (Fig. 6D, E). Then, intracellular TCRβ staining was performed using flow cytometry, and the results showed that part of NK1.1 cells express intracellular CD3/TCR after CD147 knockout (Fig. S5E). This strongly suggested that NK and NKT cells identified in tumors of CD147T-KO mice may be lineage converted from T cells. Furthermore, we examined the expression of TCRβ mRNA in CD3− NK1.1+ cells sorted from melanoma and normal spleen by qPCR. Surprisingly, although we failed to detect TCR protein, at the mRNA level there is no significant difference between wild type and CD147T-KO mice (Fig. S5F, G). We suspected that there is post-transcriptional regulation mechanism or rapid protein degradation involved to suppress TCR protein in these NK1.1+ CD3- cells. And CD147 deletion led to the number of NK1.1+ tumor infiltrating cells increasing (Table S6). To dissect out the contribution that NK-like cells had against tumor progression with CD147 deletion or functional blocking in the similar experimental melanoma model, we depleted NK-like cells through tail-vein injection of anti-NK1.1 antibody. The depletion of conventional NK, NKT and converted NK-like cells fully abolished the advantage of tumor protection in CD147T-KO mice (Fig. 6F) and WT mice under anti-CD147 treatment (Fig. 6G). These results strongly suggest that, besides its intrinsic roles in suppressing tumor progression, the clinically validated therapeutic efficacy of anti-CD147 may, at least partially, attribute to its immune modulatory function. Since the primary indication of anti-CD147 monoclonal antibody is hepatocellular carcinoma, and the liver is a unique peripheral organ that harbors both conventional and resident NK cells(Peng et al., 2013), we examined our immunomodulation hypothesis by orthotopically transplanting hepatic tumor cells that had high surface CD147 and MHC-I expression levels (Fig. S6A, B). Syngenic Hepa16 hepatoma cells carrying a constitutive active IRES luciferase reporter gene were inoculated in situ through direct intrahepatic injection. Although anti-CD147 treatment failed to show decreases in bioluminescent intensity until day 14, it ultimately suppressed the multi-organ metastasis characteristic of Hepa16 cells (Fig. 7A and data from autopsy). In the end, we observed increased survival in the anti-CD147 treatment group (Figs. 7A & S6C). In a separate experimental group, tumor-bearing mice were sacrificed for analysis at various days post tumor inoculation. Only half of the CD147T-KO mice developed tumor (Table S7). By day14, after 4 rounds of anti-CD147 antibody treatments, hematoxylin-eosin staining was performed, and indicated that the primary tumor growth was also restricted by genetic CD147 deletion or functional anti-CD147 blocking (Fig. 7B&S6G). The protection of tumor growth was further validated by calculating the liver to body weight ratio of each group (Figs. 7C & S6D). Using flow cytometry, we analyzed the dynamic changes of the NK-lineage cells in these mice and observed a steady decline of NK1.1+ thymic cells during the development of hepatoma. Interestingly, as shown with our in vitro OP9-DL1 organ cultures (Fig. 2E, F), CD147 genetic deletion and anti-CD147 treatment are both capable of increasing the thymic NK1.1+ cells (Figs. 7D & S6E), indicating that functional blockade of CD147 is sufficient to alter the lineage commitment of developing T cells in vivo. The same trend was observed in tumoral livers (Figs. 7E, S6F, H). Most importantly, in this cancer therapy model, the depletion of NK1.1+ cells in the antibody treatment group abolished the protective effect of anti-CD147 (Fig. 7C). This suggested that anti-CD147 antibody may generate professional NK-like cells for tumor surveillance.
Fig. 6.
CD147 is an immunomodulatory target for melanoma tumor therapy. 5 × 104 B16-F10 cells were transplanted subcutaneously into the thigh of mice. Starting from day 1, mice were treated with 10 mg/kg of Anti-CD147 antibody by tail vain injection every 3 days for 4 times, and tumor growth was monitored every other day starting from day 6. For the NK cell-depleting group, anti-NK1.1 antibody were given 200 ng/mouse by intraperitoneal injection every three days for four times from day 1. A. The tumor volume in WT, CD147T-KO mice, and anti-CD147 mAbs treatment mice. B. Melanoma was mechanically disrupted and digested into single cell suspension. Flow cytometry analysis of NK1.1+ cells in melanoma. C–E. qRT-PCR analysis of T-cell development related genes and NK/NKT related genes in CD8+ T cells (C) and CD3-NK1.1+ cells (D) and CD3+ NK1.1+ cells (E) from melanoma. F. The tumor volume in WT, CD147T-KO mice, CD147T-KO + anti-NK1.1 mAb treatment mice and WT + anti-NK1.1 mAb treatment mice. G. The tumor volume in WT, anti-CD147 mAbs treatment mice, anti-CD147 + anti-NK1.1 mAb treatment mice and anti-NK1.1 mAb treatment mice. Data are representative of 3 (C–E) or 5 (A–B, F–G) experiments.
Fig. 7.
CD147 is an immunomodulatory target for hepatocellular carcinoma therapy. 106 Hepa16 cells were injected into the liver of WT and CD147T-KO C57BL/6 mice in situ. Starting at day 3, the WT mice were treated with 10 mg/kg of CD147 antibody by intravenous tail vain injection, every 3 days for 2 weeks total. Six mice in the control group, six mice in the CD147T-KO group and five mice in the antibody treatment group, one mouse death due to anesthesia. A. Small animal imaging analysis was used to measure the growth of the tumor at days 3, 7, 14, 28 and 42. B. Hematoxylin-eosin staining was used to analyze tumor growth at day 14. All image analysis is shown at 100 × magnification. C. Analysis of the ratio of liver to body weight percentages at day 14 of all treatment groups. D–E. Flow cytometry analysis of NK cells in the thymus (D) and liver (E) after injection of Hepa16 cells.
4. Discussion
During T cell development, the multipotency of CLPs and LMPPs is tightly regulated at each step by sensing the thymic microenvironment through various surface receptors. Among these receptors, Notch is necessary for CLPs and LMPPs to delineate their choice of myeloid or innate lymphoid cell lineages (Yui and Rothenberg, 2014). However, our data showed that Notch signaling alone is not sufficient to carry out or maintain the T cell lineage commitment, since loss of CD147 expression or blockade of its function results in the loss of T cell identity at various stages of thymic development. Specifically, immature thymocytes that are CD147 deficient can be reprogramed to various innate lymphocytes including γδ T cells (Fig. 1), αβTCR+ PLZF+ NKT-like cells (Fig. 2), and αβTCR−/lowNK1.1+ NK-like cells (Fig. 3, Fig. 4). It is still uncertain how CD147 participates in the process of T-lineage determination. Our previous study showed that loss of CD147 in CD4+ T cells strengthens TCR activation (Yao et al., 2013). Like other inhibitory surface receptors, such as CD5, CTLA4 and PD-1, which act as a potential negative feedback blockade, surface expression of CD147 is elevated upon TCR signaling (Koch et al., 1999). Since the expression of PLZF, the master regulator for the NKT lineage, is a direct consequence of strong TCR stimulation (Seiler et al., 2012), we suspected that the observed increase of αβTCR+ PLZF+ cells might be the result of releasing pre-TCR/TCR signaling from CD147-mediated suppression. This hypothesis can also explain the observed increase of γδ T cells in CD147T-KO mice, a lineage choice which is also favored by robust receptor signaling (Hayes et al., 2010). In addition, loss of CD147 in thymocytes impairs Notch signaling, as we detected inhibited expression of Notch1 in CD147T-KO DN3 cells, as well as in genes directly regulated by Notch1 signaling such as HES1(Georgescu et al., 2008, Weng et al., 2006), DTX1 (Georgescu et al., 2008, Weng et al., 2006), and TCF1 (Taghon et al., 2005). Although CD147 has been shown to co-immunoprecipitate with γ-secretase in HeLa cells (Zhou et al., 2005), changes in CD147 expression did not impact the γ-secretase activity or Notch1 signaling (Vetrivel et al., 2008). Therefore, we do not expect that CD147 directly cross-talks with Notch pathway. On the other hand, in various lung cancer cell line cultures, overexpression of CD147 led to spontaneous Wnt/β-catenin activation (Sidhu et al., 2010). Although the contribution of canonical Wnt signaling, specifically β-catenin, in T-lineage determination remains controversial (Xue and Zhao, 2012), loss of Wnt3a impairs the DN to DP transition (Luis et al., 2009). On the background of CD147 deficiency, treatment with recombinant Wnt3a, as well as forced TCF-1 expression reversed the defects of T-lineage development. Our gain-of-function experiments indicated that CD147 might facilitate Wnt3a signaling, which is necessary for optimal activation of TCF-1, the key transcription factor in executing the function of Notch in early T-lineage specification(Weber et al., 2011). The functional roles of Wnt and Notch during development are thought to be generally divided, or even antagonizing: Wnt supports maintenance and self-renewal, while Notch is in charge of differentiation. However, at least in the case of HSCs, these two pathways seem to be integrated, since the inhibition of Notch signaling impairs Wnt signaling and the stemness of HSCs, and Wnt3a stimulation leads to the transcription of Hes1 and Dtx1 and the activation of exogenous Notch reporter (Duncan et al., 2005). A similar cooperation was also observed in vivo during vertebrate somitogenesis (Galceran et al., 2004). While the detailed molecular mechanisms are still under our investigation, we proposed that CD147 signaling may be a key mediator that links these two pathways together during early thymocyte development. As reported previously in Bcl11b deletion (Li et al., 2010b, Uddin et al., 2014), CD147T-KO mice were more resistant to B16 melanoma challenge. This resistance was diminished while the anti-NK1.1 antibody was applied to eliminate NKT, NK and NK-like cells. The enhanced anti-tumor surveillance could also be obtained by functional antibody blocking of CD147 in both the melanoma and orthotopic models of liver cancer. CD147 is an established target for antibody-mediated cancer therapy, and its clinical efficacy was attributed to the direct blockade of CD147 signaling in tumor cells (Wang et al., 2015) and antibody-dependent cell-mediated cytotoxicity (Zhu et al., 2009). However, in our animal models, the efficacy of anti-CD147 therapy also seems to rely on the presence of NK1.1+ cells. NK cells are an important population for immune surveillance against tumorigenesis, directly observed in human HCC, and recapitulated in our animal model. The reduction of NK cells (Cai et al., 2008, Guo et al., 2012) and impairment of NK functions (Cai et al., 2008, Hoechst et al., 2009) were strongly associated with tumor progression. Therefore, it is not surprising that NK-like cells generated by anti-CD147 treatment protects animal from tumor challenge. However, this study provided a new mechanistic concept that CD147 can be a target for lymphocyte lineage reprogramming. Interestingly, as discovered in ITNKs (Li et al., 2010b), NK-like cells generated from CD147 deletion do not tolerate cells with high MHC-I expression (Fig. S5B & S6B), which highlights the anti-tumor potency of this reprogrammed population and the value of CD147 as a target for clinical immune modulation.
Funding Sources
This work was supported by grants from 973 project (2015CB553704), National Science and Technology Major Project (2013ZX09301301 and 2014AA020506).
Conflicts of Interest
The authors declare no competing financial interests.
Author Contributions
JJ Geng, P Zhu and ZN Chen were responsible for overall design and execution of experiments and data analysis; JJ Geng, R chen, J Tang and XM Yang performed most experiments; Y Zhang gave assistance with Hematoxylin-eosin staining and Immunofluorescence Staining; K Zhang and JL Miao performed animal experiments; JJ Geng, P Zhu and ZN Chen wrote the manuscript.
Acknowledgments
We thank Dr. Ying Wan (the Biomedical Analysis Center of the Third Military Medical University) for providing Albino C57BL/6 mice.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.ebiom.2017.05.022.
Contributor Information
Zhi-Nan Chen, Email: znchen@fmmu.edu.cn.
Ping Zhu, Email: zhuping@fmmu.edu.cn.
Appendix A. Supplementary data
Supplementary material
References
- Als A.B., Dyrskjot L., von der Maase H., Koed K., Mansilla F., Toldbod H.E., Jensen J.L., Ulhoi B.P., Sengelov L., Jensen K.M. Emmprin and survivin predict response and survival following cisplatin-containing chemotherapy in patients with advanced bladder cancer. Clin. Cancer Res. 2007;13:4407–4414. doi: 10.1158/1078-0432.CCR-07-0109. [DOI] [PubMed] [Google Scholar]
- Biswas C., Zhang Y., DeCastro R., Guo H., Nakamura T., Kataoka H., Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995;55:434–439. [PubMed] [Google Scholar]
- Cai L., Zhang Z., Zhou L., Wang H., Fu J., Zhang S., Shi M., Zhang H., Yang Y., Wu H. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin. Immunol. 2008;129:428–437. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Caudroy S., Polette M., Tournier J.M., Burlet H., Toole B., Zucker S., Birembaut P. Expression of the extracellular matrix metalloproteinase inducer (EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary and breast lesions. J. Histochem. Cytochem. 1999;47:1575–1580. doi: 10.1177/002215549904701209. [DOI] [PubMed] [Google Scholar]
- Ciofani M., Zuniga-Pflucker J.C. The thymus as an inductive site for T lymphopoiesis. Annu. Rev. Cell Dev. Biol. 2007;23:463–493. doi: 10.1146/annurev.cellbio.23.090506.123547. [DOI] [PubMed] [Google Scholar]
- Constantinides M.G., Bendelac A. Transcriptional regulation of the NKT cell lineage. Curr. Opin. Immunol. 2013;25:161–167. doi: 10.1016/j.coi.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan A.W., Rattis F.M., DiMascio L.N., Congdon K.L., Pazianos G., Zhao C., Yoon K., Cook J.M., Willert K., Gaiano N. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat. Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- Fok K.L., Chen H., Ruan Y.C., Chan H.C. Novel regulators of spermatogenesis. Semin. Cell Dev. Biol. 2014;29:31–42. doi: 10.1016/j.semcdb.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Galceran J., Sustmann C., Hsu S.C., Folberth S., Grosschedl R. LEF1-mediated regulation of Delta-like1 links Wnt and Notch signaling in somitogenesis. Genes Dev. 2004;18:2718–2723. doi: 10.1101/gad.1249504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Radaeva S., Park O. Liver natural killer and natural killer T cells: immunobiology and emerging roles in liver diseases. J. Leukoc. Biol. 2009;86:513–528. doi: 10.1189/jlb.0309135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu C., Longabaugh W.J., Scripture-Adams D.D., David-Fung E.S., Yui M.A., Zarnegar M.A., Bolouri H., Rothenberg E.V. A gene regulatory network armature for T lymphocyte specification. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20100–20105. doi: 10.1073/pnas.0806501105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo C.L., Yang H.C., Yang X.H., Cheng W., Dong T.X., Zhu W.J., Xu Z., Zhao L. Associations between infiltrating lymphocyte subsets and hepatocellular carcinoma. Asian Pac. J. Cancer Prev. 2012;13:5909–5913. doi: 10.7314/apjcp.2012.13.11.5909. [DOI] [PubMed] [Google Scholar]
- Hahn J.N., Kaushik D.K., Yong V.W. The role of EMMPRIN in T cell biology and immunological diseases. J. Leukoc. Biol. 2015;98:33–48. doi: 10.1189/jlb.3RU0215-045R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes S.M., Laird R.M., Love P.E. Beyond alphabeta/gammadelta lineage commitment: TCR signal strength regulates gammadelta T cell maturation and effector fate. Semin. Immunol. 2010;22:247–251. doi: 10.1016/j.smim.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B., Voigtlaender T., Ormandy L., Gamrekelashvili J., Zhao F., Wedemeyer H., Lehner F., Manns M.P., Greten T.F., Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. doi: 10.1002/hep.23054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa T., Hirose S., Masuda K., Kakugawa K., Satoh R., Shibano-Satoh A., Kominami R., Katsura Y., Kawamoto H. An essential developmental checkpoint for production of the T cell lineage. Science. 2010;329:93–96. doi: 10.1126/science.1188995. [DOI] [PubMed] [Google Scholar]
- Kastner P., Chan S., Vogel W.K., Zhang L.J., Topark-Ngarm A., Golonzhka O., Jost B., Le Gras S., Gross M.K., Leid M. Bcl11b represses a mature T-cell gene expression program in immature CD4(+)CD8(+) thymocytes. Eur. J. Immunol. 2010;40:2143–2154. doi: 10.1002/eji.200940258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik D.K., Hahn J.N., Yong V.W. EMMPRIN, an upstream regulator of MMPs, in CNS biology. Matrix Biol. 2015;44–46:138–146. doi: 10.1016/j.matbio.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Kirsch A.H., Diaz L.A., Jr., Bonish B., Antony P.A., Fox D.A. The pattern of expression of CD147/neurothelin during human T-cell ontogeny as defined by the monoclonal antibody 8D6. Tissue Antigens. 1997;50:147–152. doi: 10.1111/j.1399-0039.1997.tb02853.x. [DOI] [PubMed] [Google Scholar]
- Koch C., Staffler G., Huttinger R., Hilgert I., Prager E., Cerny J., Steinlein P., Majdic O., Horejsi V., Stockinger H. T cell activation-associated epitopes of CD147 in regulation of the T cell response, and their definition by antibody affinity and antigen density. Int. Immunol. 1999;11:777–786. doi: 10.1093/intimm/11.5.777. [DOI] [PubMed] [Google Scholar]
- Kuno N., Kadomatsu K., Fan Q.W., Hagihara M., Senda T., Mizutani S., Muramatsu T. Female sterility in mice lacking the basigin gene, which encodes a transmembrane glycoprotein belonging to the immunoglobulin superfamily. FEBS Lett. 1998;425:191–194. doi: 10.1016/s0014-5793(98)00213-0. [DOI] [PubMed] [Google Scholar]
- Li L., Leid M., Rothenberg E.V. An early T cell lineage commitment checkpoint dependent on the transcription factor Bcl11b. Science. 2010;329:89–93. doi: 10.1126/science.1188989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Burke S., Wang J., Chen X., Ortiz M., Lee S.C., Lu D., Campos L., Goulding D., Ng B.L. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis T.C., Weerkamp F., Naber B.A., Baert M.R., de Haas E.F., Nikolic T., Heuvelmans S., De Krijger R.R., van Dongen J.J., Staal F.J. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- Peng H., Jiang X., Chen Y., Sojka D.K., Wei H., Gao X., Sun R., Yokoyama W.M., Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennings G.J., Kritharides L. CD147 in cardiovascular disease and thrombosis. Semin. Thromb. Hemost. 2014;40:747–755. doi: 10.1055/s-0034-1390001. [DOI] [PubMed] [Google Scholar]
- Renno T., Wilson A., Dunkel C., Coste I., Maisnier-Patin K., Benoit de Coignac A., Aubry J.P., Lees R.K., Bonnefoy J.Y., MacDonald H.R. A role for CD147 in thymic development. J. Immunol. 2002;168:4946–4950. doi: 10.4049/jimmunol.168.10.4946. [DOI] [PubMed] [Google Scholar]
- Schmitt T.M., Zuniga-Pflucker J.C. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- Seiler M.P., Mathew R., Liszewski M.K., Spooner C.J., Barr K., Meng F., Singh H., Bendelac A. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat. Immunol. 2012;13:264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu S.S., Nawroth R., Retz M., Lemjabbar-Alaoui H., Dasari V., Basbaum C. EMMPRIN regulates the canonical Wnt/beta-catenin signaling pathway, a potential role in accelerating lung tumorigenesis. Oncogene. 2010;29:4145–4156. doi: 10.1038/onc.2010.166. [DOI] [PubMed] [Google Scholar]
- Sienel W., Polzer B., Elshawi K., Lindner M., Morresi-Hauf A., Vay C., Eder F., Passlick B., Klein C.A. Cellular localization of EMMPRIN predicts prognosis of patients with operable lung adenocarcinoma independent from MMP-2 and MMP-9. Mod. Pathol. 2008;21:1130–1138. doi: 10.1038/modpathol.2008.102. [DOI] [PubMed] [Google Scholar]
- Staal F.J., Luis T.C., Tiemessen M.M. WNT signaling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8:581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- Sweeny L., Hartman Y.E., Zinn K.R., Prudent J.R., Marshall D.J., Shekhani M.S., Rosenthal E.L. A novel extracellular drug conjugate significantly inhibits head and neck squamous cell carcinoma. Oral Oncol. 2013;49:991–997. doi: 10.1016/j.oraloncology.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghon T.N., David E.S., Zuniga-Pflucker J.C., Rothenberg E.V. Delayed, asynchronous, and reversible T-lineage specification induced by Notch/Delta signaling. Genes Dev. 2005;19:965–978. doi: 10.1101/gad.1298305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Nakada M.T., Kesavan P., McCabe F., Millar H., Rafferty P., Bugelski P., Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005;65:3193–3199. doi: 10.1158/0008-5472.CAN-04-3605. [DOI] [PubMed] [Google Scholar]
- Uddin M.N., Zhang Y., Harton J.A., MacNamara K.C., Avram D. TNF-alpha-dependent hematopoiesis following Bcl11b deletion in T cells restricts metastatic melanoma. J. Immunol. 2014;192:1946–1953. doi: 10.4049/jimmunol.1301976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek S., Izon D., Hofhuis F., Robanus-Maandag E., te Riele H., van de Wetering M., Oosterwegel M., Wilson A., MacDonald H.R., Clevers H. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature. 1995;374:70–74. doi: 10.1038/374070a0. [DOI] [PubMed] [Google Scholar]
- Vetrivel K.S., Zhang X., Meckler X., Cheng H., Lee S., Gong P., Lopes K.O., Chen Y., Iwata N., Yin K.J. Evidence that CD147 modulation of beta-amyloid (Abeta) levels is mediated by extracellular degradation of secreted Abeta. J. Biol. Chem. 2008;283:19489–19498. doi: 10.1074/jbc.M801037200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yuan L., Yang X.M., Wei D., Wang B., Sun X.X., Feng F., Nan G., Wang Y., Chen Z.N. A chimeric antibody targeting CD147 inhibits hepatocellular carcinoma cell motility via FAK-PI3K-Akt-Girdin signaling pathway. Clin. Exp. Metastasis. 2015;32:39–53. doi: 10.1007/s10585-014-9689-7. [DOI] [PubMed] [Google Scholar]
- Weber B.N., Chi A.W., Chavez A., Yashiro-Ohtani Y., Yang Q., Shestova O., Bhandoola A. A critical role for TCF-1 in T-lineage specification and differentiation. Nature. 2011;476:63–68. doi: 10.1038/nature10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng A.P., Millholland J.M., Yashiro-Ohtani Y., Arcangeli M.L., Lau A., Wai C., Del Bianco C., Rodriguez C.G., Sai H., Tobias J. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L., Edwards C.K., 3rd, Zhou L. The biological function and clinical utilization of CD147 in human diseases: a review of the current scientific literature. Int. J. Mol. Sci. 2014;15:17,411–17,441. doi: 10.3390/ijms151017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H.H., Zhao D.M. Regulation of mature T cell responses by the Wnt signaling pathway. Ann. N. Y. Acad. Sci. 2012;1247:16–33. doi: 10.1111/j.1749-6632.2011.06302.x. [DOI] [PubMed] [Google Scholar]
- Yao H., Teng Y., Sun Q., Xu J., Chen Y.T., Hou N., Cheng X., Yang X., Chen Z.N. Important functional roles of basigin in thymocyte development and T cell activation. Int. J. Biol. Sci. 2013;10:43–52. doi: 10.7150/ijbs.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yui M.A., Rothenberg E.V. Developmental gene networks: a triathlon on the course to T cell identity. Nat. Rev. Immunol. 2014;14:529–545. doi: 10.1038/nri3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang Y., Sun Q., Feng F., Huhe M., Mi L., Chen Z. Preclinical pharmacokinetics, tolerability, and pharmacodynamics of metuzumab, a novel CD147 human-mouse chimeric and glycoengineered antibody. Mol. Cancer Ther. 2015;14:162–173. doi: 10.1158/1535-7163.MCT-14-0104. [DOI] [PubMed] [Google Scholar]
- Zhou S., Zhou H., Walian P.J., Jap B.K. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer's disease amyloid beta-peptide production. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7499–7504. doi: 10.1073/pnas.0502768102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Yang B., Yang X., Wang L., Xu J., Liao C., Feng Q., Tang H., Hu L., Chen Z. A novel antibody fragment targeting HAb18G/CD147 with cytotoxicity and decreased immunogenicity. Cancer Biol. Ther. 2009;8:1035–1044. doi: 10.4161/cbt.8.11.8531. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material