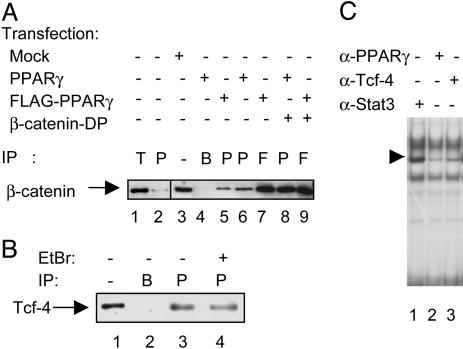
Fig. 3.
Physical interaction of PPARγ with β-catenin and Tcf-4. (A) Western blot analysis of β-catenin immunoprecipitations (IP) from the SW480 cell line. Whole-cell extracts were obtained from nontransfected cells (lanes 1 and 2), mock-transfected cells (lane 3), or cells transfected with PPARγ, FLAG-PPARγ, and/or β-catenin-DP as indicated (lanes 4-9). Lane 1 shows β-catenin coimmunoprecipitated by using an anti-Tcf-4 antibody (denoted T), whereas lane 2 shows β-catenin coimmunoprecipitated using an anti-PPARγ antibody. Lane 3 shows the endogenous β-catenin levels in SW480 cell lysates, whereas lane 4 represents a control IP with no antibody, only beads. In transfected cells, protein complexes containing β-catenin were immunoprecipitated (IP) with either a PPARγ-specific antibody (P, lanes 5, 6, and 8) or an anti-FLAG antibody (F, lanes 7 and 9). (B) Coimmunoprecipitations of endogenous Tcf-4 from the SW480 cell line. Lane 1 shows endogenous Tcf-4 levels in 25 μg of whole-cell extracts from SW480 cells, whereas lane 2 represents a mock-IP with no antibody, but with Sepharose A beads. Tcf-4 precipitated (IP) with a PPARγ-specific antibody (P) is shown in lane 3. Conditions for Tcf-4 IP in lane 4 are essentially as for lane 3, but with the addition of 10 μg/ml ethidium bromide during IP. (C) EMSA with nuclear extracts from the SW480 cells stimulated for 6 h with 20 mM LiCl. The extracts were incubated with different antibodies, as indicated, before addition of a radiolabeled PPRE probe. The major PPARγ-containing complex is indicated by the arrow.