Figure 1.
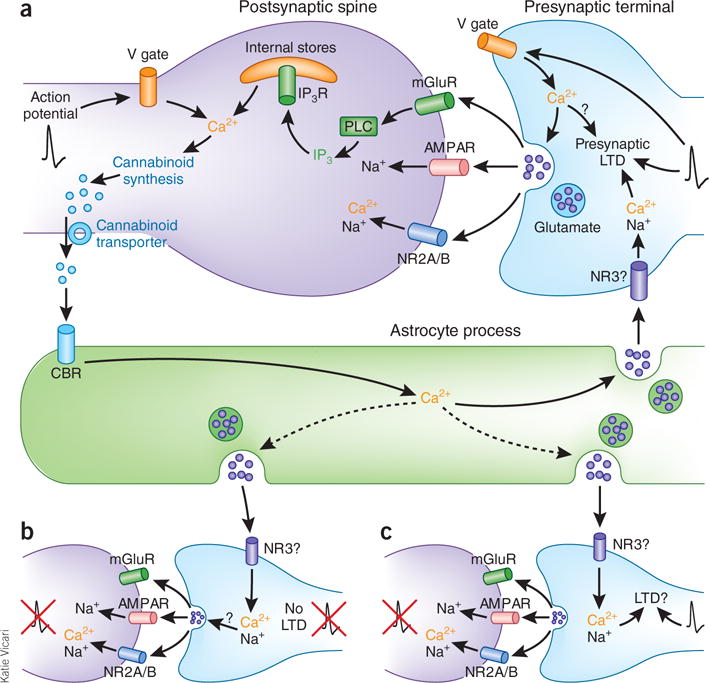
Astrocyte cannabinoid and glutamate signaling triggers tLTD at developing somatosensory cortical synapses. (a) The signaling cascade at developing L4-to-L2/3 synapses. Action potentials (black traces) at presynaptic terminals cause vesicular glutamate release, which generates the postsynaptic current mediated by AMPA-type (AMPAR) and NMDA-type (NR2A/B subunits) glutamate receptors and activates metabotropic glutamate receptors (mGluRs). The activation of mGluRs increases phospholipase C (PLC) production of inositol-1,4,5-trisphosphate (IP3), which binds to its receptor (IP3R) to release Ca2+ from intracellular stores. When a postsynaptic action potential precedes a presynaptic one by 25 ms, Ca2+ influx through postsynaptic voltage-gated Ca2+ channels (V gate) combines with Ca2+ released from intracellular stores to stimulate cannabinoid synthesis. Newly synthesized cannabinoids are transported out of the postsynaptic spine, where they activate astrocytic cannabinoid receptors (CBRs). Activation of astrocytic CBRs increases astrocyte Ca2+, which triggers vesicular release of glutamate onto presynaptic NMDA receptors (possibly the NR3 type). The activation of presynaptic NMDA receptors combined with presynaptic action potentials induces presynaptically expressed tLTD. (b,c) Although an activated astrocyte will likely release glutamate onto presynaptic NMDA receptors at other synapses it contacts, LTD will not occur at fully quiescent synapses (b) but might occur at synapses receiving presynaptic action potentials even in the absence of postsynaptic firing (c).
