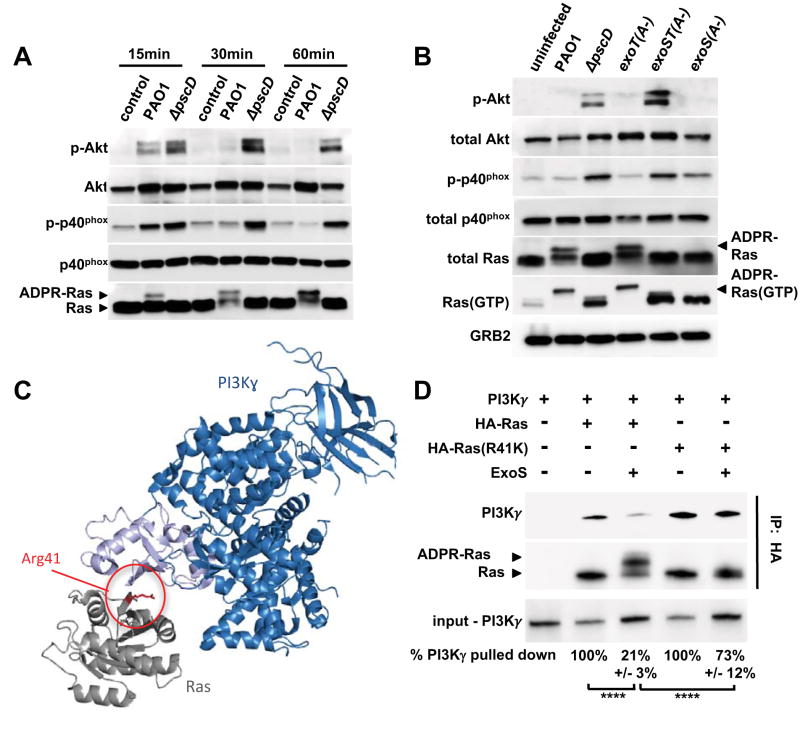
Figure 3. ExoS and ExoT ADP-ribosyltransferase activities interfere with PI3K signaling in neutrophils.
(A) Cell lysates from uninfected human neutrophils, or from neutrophils infected with wild type PAO1, or a ΔpscD mutant strain were probed by Western blot for Akt, P-Akt (Thr308), p40phox, P-p40phox (Thr154), and Ras. The experiments were repeated 3 times with similar results. (B) Cell lysates from uninfected human neutrophils, or neutrophils infected for 30 minutes with PAO1, ΔpscD, or with strains in which the ADP-ribosyltransferase activity was inactivated in ExoS only (exoS(A-)), ExoT only (exoT(A-)), or both (exoST(A-)). P-Akt (Thr308), Akt, P-p40phox (Thr154), p40phox, total Ras, GTP-bound Ras, and Grb2 (loading control) were detected by western blot. The experiments were repeated 5 times with similar results. (C) Model of Ras (gray) bound to the Ras-binding domain (light blue) of PI3K (dark blue) based on the structure PDB:1he8 (Pacold et al., 2000). Residue Arg 41 of Ras is highlighted red. (D) Purified ExoS was used to ADP-ribosylate HA-tagged versions of Ras, or Ras(R41K), in vitro and subsequently mixed with purified PI3Kγ. The interaction between Ras and PI3Kγ was probed by immunoprecipitating Ras using an anti-HA-tag antibody. PI3Kγ, as well as unmodified and ADP-ribosylated Ras were detected by western blot. The experiments were repeated 3 times with similar results. Input and output levels of PI3Kγ were determined by densitometry. The input/output ratio for the untreated control sample was set to 100% and compared to the corresponding ExoS-treatment condition (mean and standard deviation of three independent replicates are noted below each lane). Results were compared by 1-way ANOVA with Bonferroni correction (**** p<0.0001). See also Figures S3.