Abstract
Development of multifunctional biomaterials that sequester, isolate, and redeliver cell-secreted proteins at a specific timepoint may be required to achieve the level of temporal control needed to more fully regulate tissue regeneration and repair. In response, we fabricated core-shell heparin-poly(ethylene-glycol) (PEG) microparticles (MPs) with a degradable PEG-based shell that can temporally control delivery of protein-laden heparin MPs. Core-shell MPs were fabricated via a re-emulsification technique and the number of heparin MPs per PEG-based shell could be tuned by varying the mass of heparin MPs in the precursor PEG phase. When heparin MPs were loaded with bone morphogenetic protein-2 (BMP-2) and then encapsulated into core-shell MPs, degradable core-shell MPs initiated similar C2C12 cell alkaline phosphatase (ALP) activity as the soluble control, while non-degradable core-shell MPs initiated a significantly lower response (85±19% vs. 9.0±4.8% of the soluble control, respectively). Similarly, when degradable core-shell MPs were formed and then loaded with BMP-2, they induced a ~7-fold higher C2C12 ALP activity than the soluble control. As C2C12 ALP activity was enhanced by BMP-2, these studies indicated that degradable core-shell MPs were able to deliver a bioactive, BMP-2-laden heparin MP core. Overall, these dynamic core-shell MPs have the potential to sequester, isolate, and then redeliver proteins attached to a heparin core to initiate a cell response, which could be of great benefit to tissue regeneration applications requiring tight temporal control over protein presentation.
Keywords: Core-shell Microparticles, Heparin, Hydrolytically Degradable, Protein Delivery, Controlled Release
1. Introduction
Tissue regeneration is a complex process that involves intricate coordination of cellular events, many of which are modulated by proteins [1]. The concentration of these proteins, often growth factors or cytokines, must be spatially and temporally controlled to ensure proper tissue growth [2].While biomaterial-based controlled delivery systems have traditionally been used to deliver single growth factors [3, 4], tissue regeneration may require more complex temporal control over growth factor presentation and cell signaling [1]. Of particular interest, cell-secreted proteins have the potential to be captured and manipulated to enhance tissue regeneration and repair [1, 5]. For example, a protein might be locally sequestered by a biomaterial and its effect thus amplified during tissue repair [6–9]; alternatively, an undesirable protein might be captured and eliminated from the cellular microenvironment [10]. Combining these two ideas, a protein undesirable at one point in time might be captured and temporarily eliminated from the microenvironment until it is released at a second point in time, when its expression is desirable for tissue regeneration [1]. The sequestration and release of the protein of interest would be controlled both by conditions in the cellular microenvironment, such as concentration gradients [1], as well as by biomaterial properties, such as affinity of the protein to the material [11–13]. By designing materials with a specific application and protein in mind, it may be possible to precisely tune the timing of protein sequestration and release. Thus, development of dynamic biomaterials that can sequester and temporarily isolate cell-secreted proteins prior to a triggered release may achieve the level of temporal control needed to more fully regulate tissue regeneration and repair.
Core-shell MP technologies are a particularly attractive technology for temporally modulating protein presentation in the cellular microenvironment [14, 15]. Traditionally, core-shell MPs have either been used to prolong release and activity of cargo in an inner core by manipulating the degradation prolife of an outer shell [16, 17] or to sequester and concentrate various biomarkers from bodily fluids, such as plasma or urine, for diagnostic tests [18–20]. To date, only a handful of core-shell MP systems have been used to deliver proteins [14, 17, 21, 22]. In these studies, core-shell MPs were advantageous because either 1) burst release of the protein encapsulated in the core was significantly reduced due to the protective shell [14, 17, 22], or 2) the shell significantly prolonged protein bioactivity [21]. Similarly, only a few studies have shown the ability of core-shell MPs to sequester protein [18–20]. In contrast to core-shell MPs used for controlled release, core-shell MPs for biomarker sequestration are designed with an outer shell of small mesh size to exclude large proteins such as albumin, but to allow passage of smaller biomolecules, which remain trapped in the protein-binding core until retrieved for further analysis [18–20]. The two core-shell MP applications discussed above, protein delivery and biomarker sequestration, do not fully address the need for technologies able to sequester, isolate, and release cell-secreted proteins. However, taking inspiration from these technologies, it may be possible to create core-shell MPs not only to sequester proteins of interest, but also redeliver them at a user-defined time. Thus, in this work, we set out to develop a multifunctional heparin-poly(ethylene-glycol) (PEG) core-shell MP that 1) preserves protein bioactivity, 2) delivers protein in a temporally controlled manner with minimal burst release and 3) sequesters and re-delivers protein on a user-defined timescale.
A non-degradable heparin MP was used as the protein-sequestering core due to its high affinity for growth factors and ability to preserve protein bioactivity. Heparin is a highly sulfated glycosaminoglycan, often used in tissue engineering scaffolds due to its ability to bind positively charged proteins [4, 11, 13, 23–25]. Importantly, heparin can bind many growth factors involved in tissue formation, including bone morphogenetic protein-2 (BMP-2), Indian hedgehog (IHH), basic fibroblast growth factor (FGF-2), WNT, and transforming growth factor-β (TGF-β) [26]. Previous studies with these heparin MPs have shown high BMP-2 loading capacity and have suggested that BMP-2 is bioactive and can interact with cells while still bound to the MP [25]. Furthermore, heparin can protect proteins from denaturation, as it has been well documented that it protects FGF-2 from thermal and proteolytic degradation [27] and recently found that it can protect BMP-2 from degradation by heat and in aqueous solutions at physiological pH [13, 28]. Finally, these studies have shown that heparin MPs are not toxic to animals [25], which would be beneficial for future in vivo applications with these core-shell MPs. Thus, use of heparin MPs as a sequestering core could minimize burst release, present protein to cells only when the protein-loaded core is in contact with cells, and prevent protein denaturation.
PEG-diacryate (PEG-DA) was used for the degradable shell, as PEG can be chemically modified with a variety of functional groups and PEG hydrogels permit protein diffusion [29, 30]. To achieve user-defined shell degradation, dithiothreitol (DTT) was integrated into the PEG-DA network, which enhances hydrolytic degradation of the polymer network [31]. Thus, use of a PEG-DA-based shell enables protein diffusion into the heparin MP core, which then remains physically separated from cells until shell degradation.
In this work, we developed heparin-PEG core-shell MPs for potential applications in protein sequestration and subsequent re-delivery. A re-emulsification method was established to encapsulate pre-formed heparin MPs in a degradable PEG-based shell. Then, a proof-of-principle experiment was conducted to demonstrate that BMP-2-laden heparin MPs can be delivered in a temporally controlled manner to cells from core-shell MPs with a hydrolytically-degradable shell. Subsequently, it was shown that BMP-2 could be sequestered through the PEG-based shell onto the heparin core, and the BMP-2-laden heparin core could then be released to stimulate a cell response. Overall, these studies demonstrated that these core-shell MPs may provide enhanced temporal control over protein sequestration and release for potential applications in tissue regeneration and repair.
2. Materials and Methods
2.1 Polymer Synthesis
PEG-DA was synthesized according to previous methods [32]. Briefly, PEG (Sigma-Aldrich; Mn = 3.4 kDa) was reacted with acryoloyl chloride (Sigma-Aldrich) in 100% molar excess in methylene chloride (Fisher Scientific), with trimethylamine (Sigma-Aldrich) acting as a catalyst at a 1:1 molar ratio with PEG. The reaction was allowed to proceed under nitrogen purge overnight, at which point the aqueous and organic phases were separated and PEG was precipitated from the organic phase using diethyl ether (EDM Millipore) and dried. Heparin was functionalized with methacrylamide according to previous methods [8]. Briefly, the reaction was carried out in a phosphate buffer of pH 5 with 20 mg/mL heparin, 83 mM N-hydroxysulfosuccinimide sodium salt (Sigma-Aldrich), 100 mM N-(3-aminopropyl) methacrylamide hydrochloride (Polysciences), and 78 mM (N-3-Dimethylaminopropyl)-N’-ethylcarbodiimide hydrochloride (EDC) (Sigma-Alrich) for 2 hours on ice. An additional round of EDC was added, resulting in a final molarity of 156 mM. After 4 more hours, solution was dialyzed for 2–3 days and lyophilized. All polymers were stored at −20°C prior to use. To fluorescently tag heparin, heparin methacrylamide was dissolved at 10 mg/mL in 0.1 M Na2HPO4, pH 6 and reacted with Alexa-Flour (AF) 633 Hydrazide (Invitrogen) at 5.7 µM concentration with 0.1 M EDC for 1 hour. The solution was dialyzed for 2 days and lyophilized. Proton NMR (1H NMR) was used to determine the percent functionalization of heparin methacrylamide as previously described [25]. Briefly, Heparin was dissolved at 10 mg/mL in deuterated water and run on a Bruker Avance III 400 spectrometer. The percent of carboxyl groups substituted with methacrylamide groups was determined by comparing the integration regions of N-(3-aminopropyl) methacrylamide hydrochloride and unmodified heparin.
2.2 Microparticle Fabrication
2.2.1 Heparin MP Fabrication
Heparin methacrylamide MPs were formed according to previous methods [25]. Briefly, an aqueous phase of 10% heparin methacrylamide (wt%), 18 mM ammonium persulfate (Sigma-Alrich), and 18 mM N,N,N′,N′-Tetramethylethylenediamine (Sigma) were emulsified against corn oil with 1.67% (v/v) Tween-20 (polysorbate 20; BDH) at a 1:120 ratio aqueous:oil phase. MPs were cross-linked under nitrogen purge at 60°C for 30 minutes, then washed with acetone and water. Heparin MPs were filtered using a size extrusion device (Lipex Thermoline extruder, Northern Lipids) against a 12 µm nucleopore membrane to ensure MPs were all less than12 µm in diameter. For Alex Flour (AF)633 tagged heparin MPs, the same procedure was followed but tagged heparin was included at 70 wt% total polymer content. All MPs were stored in PBS at 4°C prior to use. To measure the mass of heparin MPs, an aliquot was flash-frozen in liquid nitrogen and lyophilized overnight. By weighing the test tube prior to adding the aliquot and after lyophilizing, the dry mass of heparin MPs was determined.
2.2.2 Core-Shell and PEG-Based MP Fabrication
Degradable and non-degradable core-shell MPs were formed by suspending fully hydrated and swollen pre-formed heparin MPs in a precursor aqueous phase containing 16 wt% PEG-DA, 0.05 wt% Irgacure 2959 Photoinitiator (Ciba), 2 mg/mL poly-L-lysine (PLL, Sigma-Aldrich), 45 mM DTT (degradable MPs only) and 0.33 mg/mL FITC-PEG-SH (1 kDa; NANOCS; for fluorescently tagged MPs only). Throughout the text, core-shell MPs indicate non-degradable MPs, while degradable core-shell MPs indicate degradable MPs. For MPs with FITC-PEG or DTT, the aqueous phase was allowed to incubate for 1 hour at 37°C to allow for a click reaction to occur between the thiolated FITC molecule or DTT and the acrylate group on the PEG molecules. The aqueous phase was then emulsified with a homogenizer against a mineral oil phase (light, white; Ameresco) with 0.3–1.3% (v/v) Span-80 (sorbitan monooleate; TCI) at a 1:16.7 ratio aqueous:oil phase, nitrogen purged for 1 minute, then cross-linked under UV light (approximately 10.5 mW/cm2) in a 35×10 mm petri dish for 10 minutes. Core-shell MPs were then centrifuged at 10,000 RCF for 5–10 minutes, washed with water twice, and filtered using the size extrusion device against a 12 µm filter to remove any remaining free heparin MPs. PEG-based MPs were made in a similar fashion to the core-shell MPs, but without heparin MPs in the aqueous phase. All MPs were stored in PBS at 4°C prior to use.
2.3 Core-Shell Microparticle Characterization
2.3.1 Quantification of Heparin MPs in Core-Shell MPs
To characterize core-shell MPs, fluorescent core-shell MPs were imaged via confocal microscopy. Heparin methacrylamide tagged with AF633 was used to fabricate the heparin MP core and FITC-PEG-SH was included in the aqueous PEG phase. Confocal microscopy (20x objective, LSM 700; Zeiss) was used to image stacks of MPs at 2 µm intervals. To immobilize MPs for imaging studies, core-shell MPs were suspended in a 10 wt% PEG-DA (8kDa) phase with 0.05% D2959 and cross-linked under UV light (approximately 10.5 mW/cm2) in PTFE (Teflon) wells for 10 minutes to form MP-containing hydrogels discs of approximately 6 mm in diameter and 1 mm thick. This kept MPs from drifting while confocal stacks were being taken. Stacks were then z-projected and orthogonal views were used to confirm complete encapsulation of heparin MPs within the PEG-based shell.
To quantify number of heparin MPs/core-shell MP, ImageJ was used to z-project and split stacks into FITC (green, PEG-based shell) and AF633 (red, heparin core) channels. Then, each PEG-based shell was defined as a Regions of Interest (ROI) through thresholding and particle analysis of the FITC channel. Next, the number of heparin MPs in each ROI was counted using thresholding and particle analysis of the AF633 channel. Finally, number of heparin MPs per each core-shell MP was graphed against its cross-sectional area (CSA; µm2). Linear correlations were obtained for masses of 0.1, 0.25, 0.5. 0.75, and 1 mg heparin MPs suspended in precursor PEG phase and each mass was tested in three separate MP batches (n=3 batches, 50–100 MPs analyzed/batch).
In all subsequent studies, a mass of 1 mg heparin MPs was used. For each batch of core-shell MPs used for protein pull-down studies and cell studies, core-shell MPs were sized and a histogram of core-shell CSA was constructed with binning at every 40 µm2. The average slope of the lines of the linear correlations obtained for a mass of 1 mg was then used to determine an average number of heparin MPs/core-shell MP based on average number of core-shell MPs per each bin. With this information, the correct number of core-shell MPs could be used to match the mass of heparin in the core-shell MPs to the mass of the heparin MP controls.
2.3.2 MP Degradation Studies
For core-shell and PEG-based MP degradation studies, MPs were counted with a hemocytometer and incubated at concentration of 1 million MPs/mL in PBS at 37°C on a shaker plate at 65 RPM (Barnstead Lab-Line, Multipurpose Rotor). 30 µL aliquots of MP solution was taken every 2–3 days and imaged using phase microscopy. Core-shell MPs were determined to be degraded when few (less than 10 MPs) or no core-shell MPs were visible.
2.4 Protein Loading and Release
2.4.1 BMP-2 and SDF-1α Protein Pull-Down
Degradable core-shell MPs and PEG-based MPs (45 mM DTT) with 1 mg heparin were prepared. The total mass of heparin MPs was 0.02 mg for both the degradable core-shell MPs and the heparin MPs. The quantification method was used to determine the number of core-shell MPs required to obtain 0.02 mg heparin/sample, and the number of degradable PEG-based MPs was matched to the number of degradable core-shell MPs. 90 ng of recombinant human BMP-2 or stromal cell-derived factor-α (SDF-1α) (R&D Systems) was loaded onto the MPs in 1 mL 0.1% bovine serum albumin (BSA) PBS solution in low-binding tubes, resulting in 4.5 µg protein/mg heparin MP. MPs were incubated at 4°C on rotary for 2 or 24 hours, at which point they were centrifuged at 10,000 RCF for 3 minutes and the supernatant was analyzed using an enzyme-linked immunosorbent assay (ELISA) per the manufacturer’s protocol ( Human BMP-2 DuoSet ELISA DY355 and Human CXCL12/SDF-1 DuoSet ELISA DY350, R&D Systems). Briefly, in these sandwich ELISAs, a primary antibody was plated, followed by the sample, then a biotinylated secondary antibody, followed by an avidin-linked horse-radish peroxidase (HRP) enzyme, and finally a substrate solution that generated a color that can be read on a plate reader at 450 nm.
2.4.2 BMP-2 Loading and Release
Two techniques were used for loading core-shell MPs. In the first technique, heparin MPs were loaded with protein prior to encapsulation into the PEG shell (“pre-fabrication load”; Fig. 4A). In the second technique, core-shell MPs were formed and then loaded with protein (“post-fabrication load”; Fig. 6A). Because small proteins are able to diffuse through PEG-DA networks [29], it was hypothesized that encapsulated heparin MPs would still be able to sequester protein, which was confirmed in the pull-down studies discussed in Section 2.4.1.
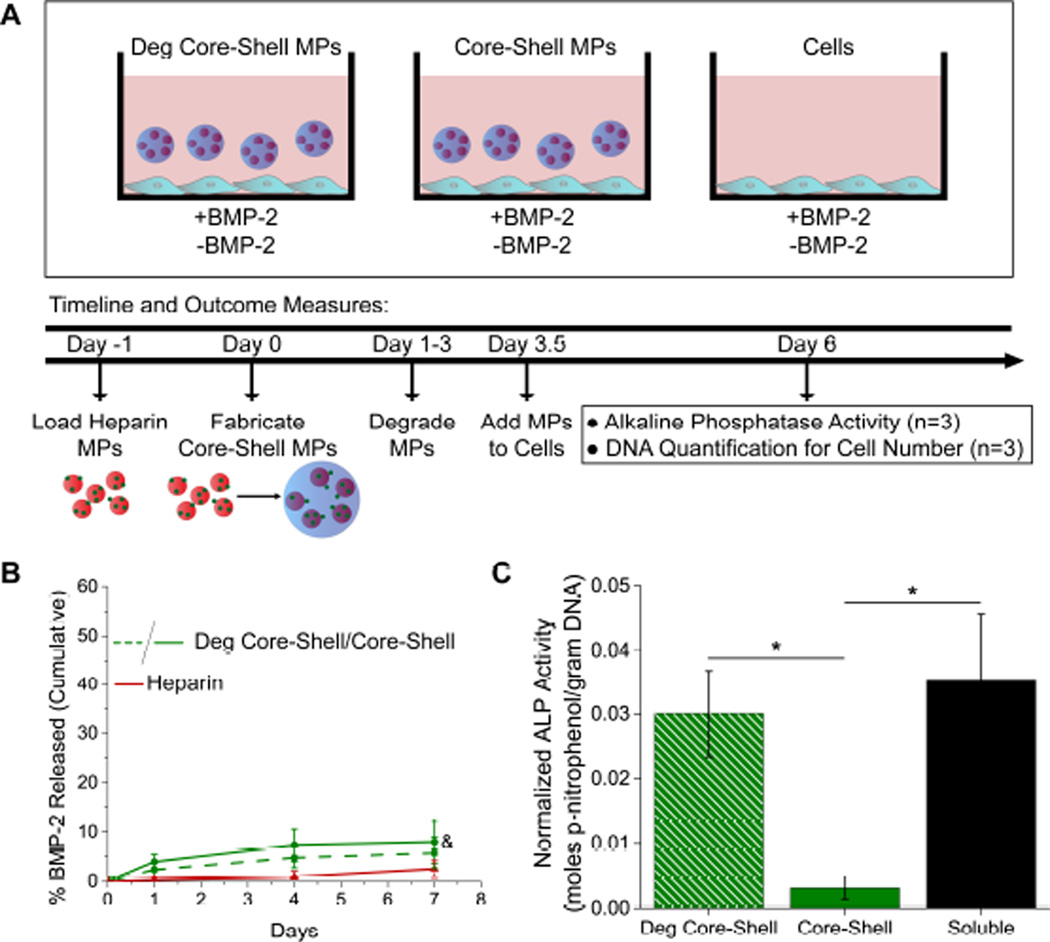
Figure 4.
Degradable (Deg) core-shell MPs modulate loaded heparin MP delivery to cells. (A) Experimental set-up for pre-fabrication loaded core-shell MPs. (B) Cumulative percent released of loaded BMP-2 for deg core-shell, core-shell, and heparin MP groups (&=significantly different from heparin MP group, p<0.05, n≥3). (C) Normalized ALP activity for deg core-shell, core-shell, and soluble BMP-2 groups (non-loaded groups showed no signal; *=significantly different from core-shell MP group, p<0.05, n=4).
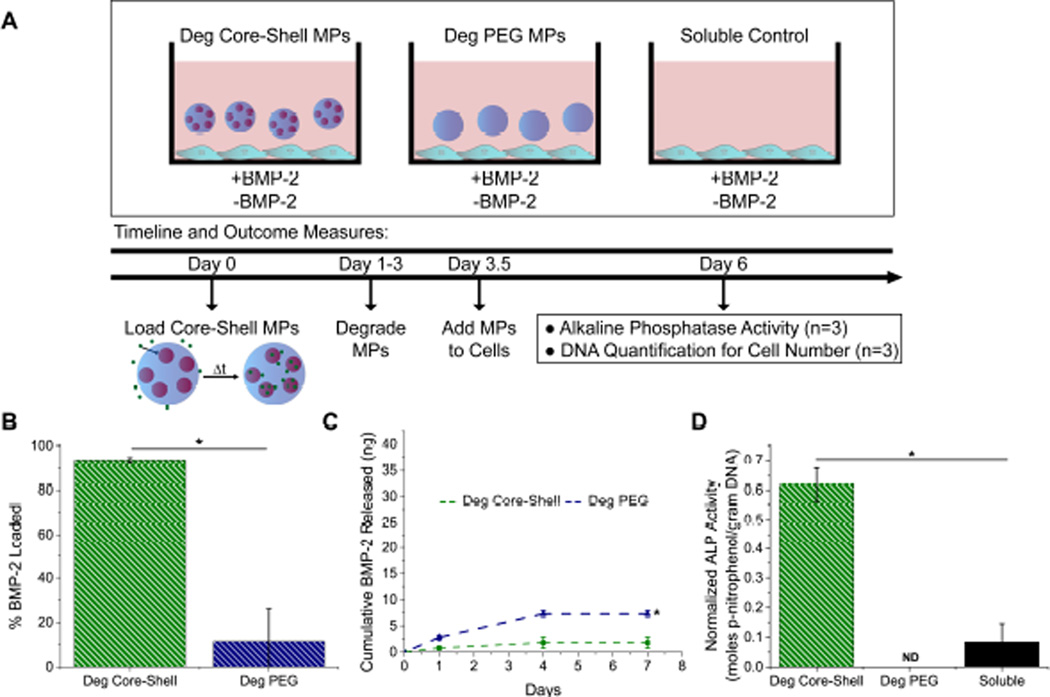
Figure 6.
Post-fabrication loading allows for delivery of loaded heparin MPs. (A) Experimental set-up for post-fabrication loaded MPs. (B) Post-fabrication loading resulted in nearly 100% loading in deg core-shell MP group and less than 10% loading in deg PEG-based MP group. (C) Cumulative mass of BMP-2 released for deg core-shell and deg PEG-based MPs for seven days. (D) Normalized ALP activity for deg core-shell MP, deg PEG-based MP, and soluble groups. “ND” indicates not detectable (non-loaded groups showed no signal) (*=Significantly different from deg core-shell group; p<0.05, n=4).
For “pre-fabrication load” studies, 1 mg heparin MPs were loaded at a concentration of 1.5 µg BMP-2/mg MP in 1 mL 0.1% BSA solution in low-binding tubes at 4°C on rotary overnight. MPs were centrifuged at 10,000 RCF for 3 minutes and supernatant was removed and analyzed using an ELISA (Human BMP-2 DuoSet ELISA DY355,R&D Systems) to calculate total BMP-2 loaded. Then, loaded heparin MPs were used to form degradable and non-degradable core-shell MPs with 1 mg heparin MPs. Using the quantification method for core-shell MPs, degradable core-shell MP, core-shell MP, and heparin MP groups all contained 0.02 mg heparin MPs and were incubated in 0.5 mL 0.1% BSA solution in PBS at 37°C for seven days on a shaker plate. At days 1, 4, and 7, MPs were centrifuged at 10,000 RCF for 3 minutes and 0.5 mL supernatant was removed for analysis and replaced with 0.5 mL fresh 0.1% BSA solution. Supernatant was analyzed using an ELISA (Human BMP-2 DuoSet ELISA DY355, R&D Systems).
For “post-fabrication load” studies, degradable core-shell and PEG-based MPs were fabricated with 1 mg heparin MPs. Using the quantification method for core-shell MPs, degradable core-shell MP groups contained 0.02 mg heparin MPs. The number of PEG-based MPs and degradable core-shell MPs were matched. All MPs were loaded at a concentration of 4.5 µg BMP-2/mg heparin MP in 0.5 mL 0.1% BSA PBS solution in low-binding tubes at 4°C on rotary overnight. MPs were centrifuged at 10,000 RCF for 3 minutes and 0.5 mL supernatant was removed and analyzed using an ELISA (Human BMP-2 DuoSet ELISA DY355, R&D Systems) to calculate total BMP-2 loaded. Supernatant was replaced with a fresh 0.5 mL 0.1% BSA solution in PBS and MPs were incubated at 37°C for seven days on a shaker plate. At days 1, 4, and 7, MPs were centrifuged at 10,000 RCF for 3 minutes and 0.5 mL supernatant was removed for analysis and replaced with 0.5 mL fresh 0.1% BSA solution. Supernatant was analyzed using an ELISA (Human BMP-2 DuoSet ELISA DY355, R&D Systems).
2.5 Alkaline Phosphatase Activity Assays
2.5.1 C2C12 Cell Culture
The C2C12 cell line was used to evaluate the ability of core-shell MPs to delivery bioactive BMP-2 after shell degradation, as C2C12 cells produce Alkaline Phosphatase (ALP) in response to BMP-2 [33]. C2C12 cells were cultured at 37°C, 5% CO2 in DMEM with 4.5 g/L glucose, fetal bovine serum (FBS) (10% for growth media, 1% for assay media; Hyclone), 2 mM L-glutamine, 50 IU Penicillin and 50 µg/mL Streptomycin. For assays, cells were plated at 62,500 cells/cm2 in 96 well plates and were incubated for 6 hours to allow adherence before the assay was started.
2.5.2 Microparticle Preparation
Microparticles were prepared for pre- or post-fabrication load experiments as described in section 2.4.2 and were sterilized by washing with 70% ethanol for 30 minutes, followed by 3 washes with PBS for 30 minutes each. MPs were centrifuged at 10,000 RCF for 3 minutes and supernatant was removed between each sterilization and wash. For pre-fabrication studies, groups included pre-fabrication loaded and non-loaded degradable core-shell MPs, pre-fabrication loaded and non-loaded core-shell MPs, and a soluble BMP-2 and a no BMP-2 media control (Fig. 4A). For post-fabrication studies, groups included post-fabrication loaded and non-loaded degradable core-shell microparticles, loaded and non-loaded degradable PEG-based MPs, and a soluble BMP-2 and no BMP-2 control (Fig. 6A). All MPs were incubated for 2.5 days prior to being added to cells for pre-degradation. This allowed MP shell degradation to occur during the three day cell assay (while degradation occurs throughout the entire time course, the majority of the PEG-based MP degradation occurred between days 3–6 for these MPs; Supplementary Fig. 1B and 2). All core-shell and heparin MP groups had 0.02 mg of heparin and the degradable PEG-based control group had the same number of MPs as the core-shell group. Cells were cultured for 3 days and then media/MPs were removed.
2.5.3 Cell lysis, ALP Activity, and DNA content
After media and MP removal, cells were washed twice with PBS. DdH2O was added to cells for 20 minutes and then cells were subjected to a freeze-thaw cycle. Cells were then scraped from the 96-well plate and cell lysate solution was transferred to 1.7 mL tube. Cell lysate was sonicated for 20 minutes, then subjected to another freeze-thaw cycle. This was repeated once, then samples were centrifuged at 10,000 RCF for 5 minutes and supernatant was used for analysis. For ALP activity, 50 µL sample was combined with 50 µL 1.5M 2-amino-2-methyl-1-propanol (Sigma-Aldrich), 50 µL 10 mM magnesium chloride, and 50 µL 20 mM p-nitrophenol phosphate disodium salt hexahydrate (Sigma-Aldrich). For standards, p-nitrophenol (Sigma-Aldrich) was used. Samples were allowed to incubate for 2 hours and absorbance was read at 405 nm. DNA content was assessed with the CyQUANT Assay following the manufacturer’s instructions and using bacteriophage λ DNA to create a standard curve (ThermoFisher Scientific). The assay was read at excitation/emission of 480/520.
2.6 Statistical Analysis
All results are depicted as mean ± standard deviation. Analysis of Variance (ANOVA) was used to identify significant factors and interactions, then Tukey’s post hoc test (significance level p ≤ 0.05) was used to generate pairwise comparisons between means of individual sample groups and determine statistically significant differences (Minitab 15 Statistical Software).
3. Results
3.1 Core-Shell MP Fabrication and Characterization
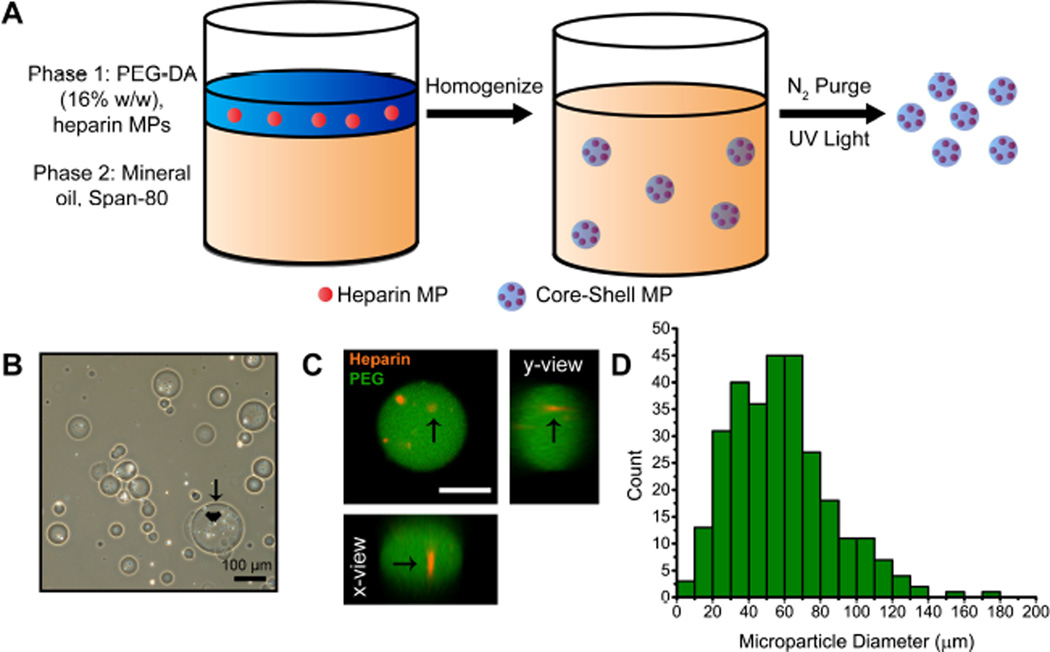
Core-shell MPs were fabricated by suspending heparin MPs (15–27% methacrylamide functionalization, 2.4±1.8 µm in diameter) of known mass in a precursor aqueous PEG-DA phase that was emulsified against mineral oil and then cross-linked via free radical initiated polymerization (Fig. 1A). The size of core-shell MPs could be controlled by varying the concentration of surfactant (Span-80) in the oil phase (Supplementary Fig 1A). Heparin MPs appeared encapsulated inside PEG-based shell in phase microscopy images (Fig. 1B) and encapsulation was confirmed using orthogonal views of three-dimensional image stacks from confocal microscopy (Fig. 1C). A histogram was constructed by combining three different batches of core-shell MPs with 1 mg of heparin MPs, and the average core-shell MP diameter was 58±28 µm (Fig. 1D).
Figure 1.
(A) Core-shell MPs were formed via a water-in-oil emulsion. (B) Phase images of core-shell MPs. Arrows and arrowheads indicate the PEG-based shell and heparin core, respectively. (C) Orthogonal view from 3D confocal image stacks confirmed encapsulation of heparin core (red, arrow) into PEG-based shell (green) (scale bar = 25 µm). (D) Histogram of size distribution for core-shell MPs with 1 mg heparin MPs. The average core-shell MP diameter was 58±28 µm and the median 55 µm.
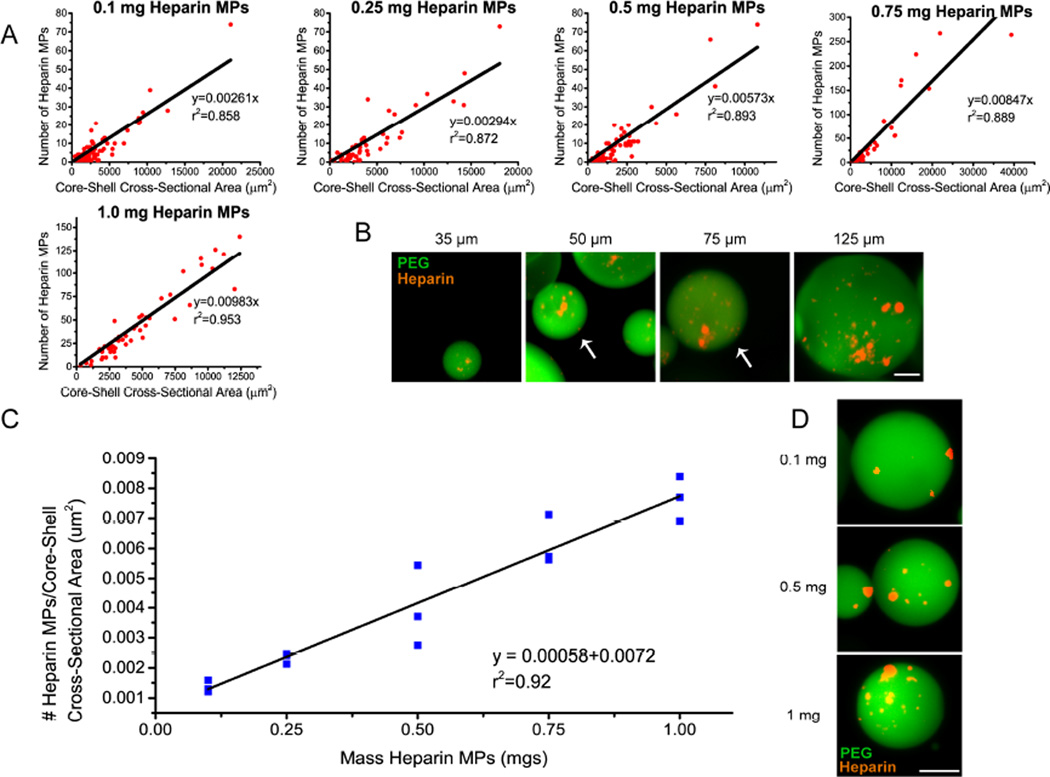
As multiple heparin MPs are encapsulated in each PEG-based shell in this fabrication process, the number of heparin MPs per core-shell MP was determined in order to achieve known heparin dosages for subsequent cell studies. Using ImageJ, the number of heparin MPs per core-shell MP was determined and a linear correlation was found between the number of heparin MPs and the CSA of each core-shell MP (Fig. 2A). As the CSA of the core-shell MP increased, the number of heparin MPs increased (Fig 2A and B). The ratio of the number of heparin MPs:core-shell CSA also increased as the mass of heparin MPs in the pre-cursor was increased, evidenced by the increasing slope in graphs of heparin MPs vs. core-shell CSA (Fig 2A). A second linear correlation was found between mass of heparin MPs in precursor PEG phase and the ratio of heparin MPs:core-shell CSA (Fig. 2C). Thus, for similarly sized core-shell MPs, the number of heparin MPs increases as the mass of heparin MPs in the pre-cursor PEG phase increases (Fig. 2C and 2D).
Figure 2.
The heparin content in core-shell MP is correlated to MP size and mass of heparin MPs in the precursor PEG solution. (A) Number of heparin MPs correlated linearly with core-shell MP cross-sectional area for five masses of heparin tested. Note axes are different in 0.75 and 1 mg groups due to increases in MP size (n=3 batches of MPs for each mass). (B) Representative images of MPs fabricated with 1 mg of heparin ranging from 35–125 µm in diameter, noted above each image and pointed out by white arrows if more than one MP/image (PEG in green, heparin in red ; scale bar = 25 µm). (C) The ratio of heparin MPs/core-shell size increases as the mass of heparin MPs in precursor PEG phase is increased (n=3 batches of MPs for each mass). (D) Representative images of 0.1, 0.5, and 1 mg heparin MP encapsulated in PEG shell (scale bar = 25 µm).
3.2 Core-Shell MP Degradation
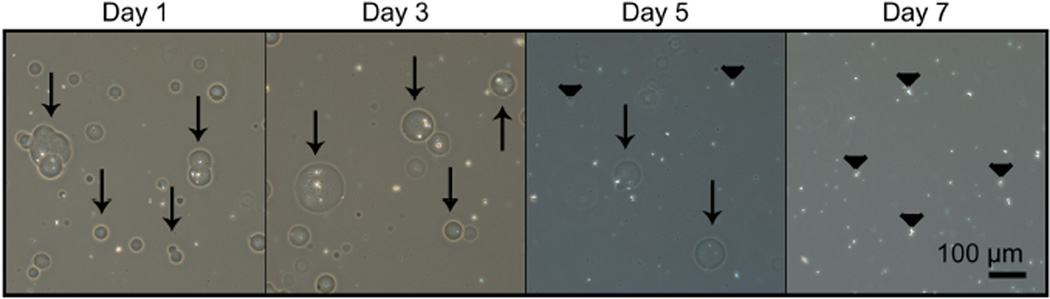
Hydrolytic degradation of PEG-DA MPs can be accelerated by adding DTT into the network, which enhances the susceptibility to hydrolysis of the ester bond [31]. By varying the concentration of DTT, degradation rate of PEG-based MPs could be temporally modulated, allowing a range of degradation time between 8 and 23 days (Supplementary Fig. 1B). For core-shell MPs, DTT was incorporated into the PEG-DA network at a 45 mM concentration (corresponding to a molar ratio of 1.21:1 PEG-DA:DTT) and the shell of the core-shell MPs were observed to degrade in approximately six days using phase microscopy. In addition, the non-degradable heparin MP core was released and present after shell degradation (Fig 3).
Figure 3.
Degradation of PEG-based shell and release of heparin MP core from core-shell MPs. Core-shell MPs are present through day 5 (days 1–5, arrows), at which point they begin to degrade and release heparin MPs (days 5 and 7, arrowheads). Core-shell MPs are fully degraded by day 7.
3.3 Pre-Fabrication Load Bioactivity Studies
For pre-fabrication load studies, heparin MPs loaded 98.0±0.1 % of BMP-2. Degradable and non-degradable core-shell MPs were fabricated with loaded heparin MPs and release of BMP-2 was monitored over seven days. As BMP-2 binds tightly to heparin MPs, very little BMP-2 was released over the course of seven days (5.8±3.2%, 7.9±4.3%, and 2.5±1.7% for degradable core-shell, core-shell, and heparin MPs, respectively; Fig. 4B). Degradable core-shell microparticles induced similar C2C12 ALP activity as the soluble control, while core-shell microparticles induced significantly lower activity after three days (85±19% vs. 9.0±4.8% of soluble control, respectively; Fig. 4C). No signal was observed for groups without BMP-2.
3.4 Post-Fabrication Load Protein Sequestration Studies
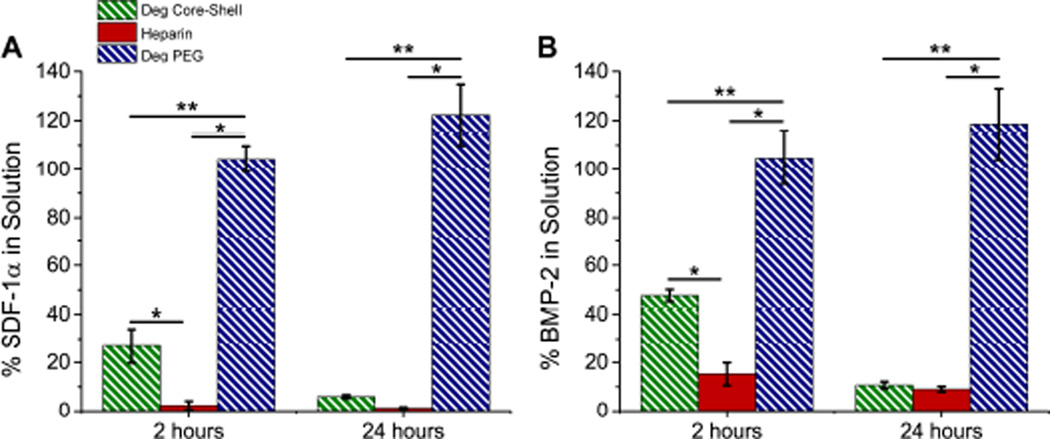
When degradable core-shell MPs were loaded with BMP-2 and SDF-1α, sequestration of proteins was temporally delayed. At two hours, significantly more SDF-1α remained in solution after incubation with core-shell MPs than with heparin MPs (26.8±7.1% and 2.3±1.8%, respectively). However, by twenty-four hours similar amounts of protein were found for each group (6.0±0.7% and 1.2±0.4%, respectively; Fig. 5A). BMP-2 behaved similarly, with significant differences in sequestration observed at 2 hours (47.8±2.4% and 15.2±4.6% remaining for core-shell and heparin MPs, respectively) but similar levels of sequestration by twenty-four hours (10.7±1.4% and 9.0±1.2% remaining for core-shell MPs and heparin MPs, respectively; Fig. 5B). In both cases, no protein sequestration was observed in the PEG-based shell group. All results are normalized to the soluble control group. These studies indicate that it is possible to sequester protein through the PEG-based shell of core-shell MPs, but that sequestration is temporally delayed. Thus, for all post-fabrication loading cell studies, a 24 hour loading time was used.
Figure 5.
Core-shell MPs temporally modulate protein sequestration. Protein pull-down studies with growth factors (A) SDF-1α and (B) BMP-2. Graphs show percent of protein remaining in solution (i.e. percentage of protein not sequestered by MPs) normalized to soluble protein controls. (*=significantly different from heparin MP group, **=significantly different from degradable core-shell; p<0.05, n≥3).
3.5 Post-Fabrication Load Bioactivity Studies
Loading studies indicated that degradable core-shell MPs loaded 93.5±1.0% of BMP-2 while degradable PEG MPs loaded only 11.5±14.6% as compared to the soluble control (Fig. 6B). Core-shell MPs released only 2.7±1.6% of loaded BMP-2 over seven days (Fig. 6C). Degradable core-shell MPs induced ~7-fold higher C2C12 ALP activity than the soluble control, while no detectable signal was observed for the loaded degradable PEG-based MP group (Fig. 6D). No signal was observed for groups without BMP-2.
4. Discussion
In these studies, core-shell MPs were designed to sequester, isolate and protect, and deliver protein on a user-defined timescale (Fig. 1A). Previously, a variety of fabrication techniques have been employed to make core-shell MPs. The double emulsion fabrication technique is a convenient, one-batch reaction, it requires two immiscible phases [34–37], restricting the chemical properties of the materials that can be used. Layer-by-layer coating technqiues can provide control over shell size, but require layers to interact with each other through electrostatic interactions [21], again narrowing material choice to those with positive or negative charges. Because the heparin-PEG core-shell MPs presented here required use of specific materials, each having a separate function, a fabrication technique that permitted greater flexibility in material choices was required. Thus, a re-emulsification technique was chosen [38] for core-shell MP fabrication to allow use of heparin and PEG-based materials. In this method, pre-formed heparin MPs were re-emulsified in a precursor PEG phase (Fig. 1A), a process mild enough to maintain protein bioactivity when protein was loaded onto heparin MPs pre-fabrication (loading of heparin MPs prior to re-emulsification; Fig. 4C).
Core-shell MP characterization revealed that the number of heparin MPs was linearly correlated to the CSA of core-shell MPs, and that increasing the mass of heparin in the precursor PEG phase increased the number of heparin MPs encapsulated (Fig. 2). Thus, although a heterogeneously-sized population of MPs was formed in each batch (Fig. 1D), the number of heparin MPs per core-shell MP was tunable and predictable. If more homogenously-sized MPs per batch are required for future experiments, this re-emulsion technique could be translated to a microfluidic device to increase core-shell MP size homogeneity [35], as a PEG-based MPs have previously been fabricated on microfluidic devices with a variety of cross-linking techniques [39–41].
Recently, it has been shown that hydrolytic degradation can be enhanced in PEG-DA hydrogels by integrating DTT into the polymeric network [31]. In this work, slow- and fast-degrading MPs were developed by varying the amount of DTT added to the aqueous PEG phase (Supplementary Fig. 1B). Previously, DTT has been incorporated into PEG-DA MPs using a water-in-water emulsion technique, but it was shown that increasing the concentration of DTT resulted in increased MP size, likely due to increased chain length and consequently, a larger mesh size [42]. To overcome this limitation, a water-in-oil emulsion technique was used, as MP size could be modulated independently of DTT content by varying surfactant concentration (Supplementary Fig. 1A). Thus, tunable degradation and controllable size of the PEG-based MP shells was achieved, which was then applied to core-shell MPs to enable shell degradation in approximately six days (Fig. 3).
In these experiments, core-shell MPs were loaded using two different techniques. In the first, a “pre-fabrication loading” technique (Fig. 4A), heparin MPs were loaded with BMP-2 prior to encapsulation into the PEG-based shell. Using the pre-fabrication loading technique may be advantageous because the core-shell fabrication technique does not require use of solvents and heparin can protect proteins from denaturation [13, 27, 28], thus maintaining the bioactivity of loaded proteins. Furthermore, pre-fabrication loading could allow for loading of proteins in both the heparin core and PEG-based shell in future experiments for dual-release applications. When core-shell MPs were pre-fabrication loaded with BMP-2, degradable and non-degradable core-shell MPs released very little protein over the course of seven days (5.8±3.2%, 7.9±4.3%, and 2.5±1.7% for degradable core-shell, core-shell, and heparin MPs, respectively; Fig. 4B). These results are comparable to what has been seen in previous studies when heparin MPs loaded with 1000 ng BMP-2/mg heparin MPs showed less than 10% release of BMP-2 after 30 days [25].
Because so little free protein is released from the core-shell MPs, only degradation of the PEG-based shell and release of the protein-laden heparin core would be able to initiate a response from C2C12 cells. This necessitates the ability of protein to interact with cell receptors while still bound to heparin. Here and in past studies [25], it was evident that BMP-2 could interact with cells while bound to the heparin MPs (Fig. 4C and 6D), possibly with greater efficiency, as ALP activity was higher in groups with BMP-2 loaded on core-shell MPs than soluble BMP-2 (Fig. 6D). In addition, evidence from in vivo and in vitro studies indicates that many other proteins, such as IHH, FGF, and WNT can also interact with their cell surface receptor while bound to heparin or heparan sulfate, potentially with enhanced affinity [2, 43], indicating that this core-shell technology is versatile and can be used for delivery of a variety of heparin-binding proteins.
Pre-fabrication loaded degradable core-shell MPs were able to initiate a cell response that was similar to the response initiated by the soluble control, unlike the non-degradable control group, which demonstrated no cellular response (Fig. 4C). This is distinctly different than many other delivery systems, which generally have an unavoidable burst release prior [15, 17, 44]. Here, protein release from a loaded vehicle was prevented for over three days in the non-degradable group, strongly supporting the idea that in this system, the majority of protein presentation can only occur after shell degradation and release of the protein laden core. By tuning the degradation rate of the PEG-based shell, it would be possible to further adjust the delay period between MP delivery and protein presentation, beneficial for applications that require delivery of different proteins at different points in time. Specifically, optimization of cellular differentiation processes such as chondrogenesis for cartilage regeneration [45] or myogenesis for muscle regeneration [46] may benefit from such a technology that promotes complex temporal coordination of protein presentation. In addition, tissue repair processes such as wound healing and vascularization could benefit from release vehicles with tight temporal control over protein release [1].
For the second loading technique employed with these core-shell MPs, “post-fabrication loading,” core-shell MPs were formed and then loaded with BMP-2 by diffusion through the outer shell (Fig. 6A). First, protein sequestration studies were conducted to determine if protein diffusion through the PEG-based shell could occur, using two different model proteins, SDF-1α and BMP-2, chosen because they possess molecular weights (~7–16 kDa) that represent small to average size for many growth factors, including but not limited to BMP-2, IHH, FGF-2, and TGFβ-1 (molecular weights ranging from 12–20 kDa, R&D Systems website [47]). Interestingly, a slight temporal delay in sequestration of both proteins was observed in the degradable core-shell MP groups as compared to the heparin MP control, indicating that the PEG-based shell delayed diffusion of these proteins. Thus, for the rest of post-fabrication load studies, core-shell MPs were incubated with protein for 24 hours to ensure complete protein loading.
These findings are similar to previous studies that have shown that protein diffusion is slowed but not inhibited by a PEG-DA hydrogel network [29, 48, 49]. For example, one study found that myglobin (17 kDa, similar in size to the proteins used in this study) was able to diffuse through both 2 and 10 kDa PEG networks, but at a slower rate in the 2 kDa network [29]. (NB: Because PEG may protect protein from denaturation in aqueous solution [37], protein levels were observed to be greater than 100% of soluble control (Fig. 5)). As the rate of protein diffusion is controlled by the hydrogel network pore size, diffusion rates can be further modulated by changing the MP pore size. In these core-shell MPs, pore size can be controlled by adjusting the molecular weight of PEG and the total DTT content in the network [42], a strategy that can be used in future work aiming to more tightly control protein diffusion.
After loading with BMP-2, very little release was observed in post-fabrication loaded degradable core-shell MPs over the course of 7 days (2.7±1.6% of loaded BMP-2), similar to what was seen in pre-fabrication loading release studies. In a proof-of-principle study, when incubated with C2C12 cells, post-fabrication loaded degradable core-shell MPs induced ~7-fold higher C2C12 ALP activity than the soluble control (Fig. 6D), indicating that these MPs can effectively sequester and then re-present growth factors to cells. The degradable PEG-based MP control, in which no signal was detected, was used to ensure that cell response was not due to free BMP-2 caught in the PEG network during loading. In uptake studies, it was found that heparin MPs were internalized by cells, in what appeared to be a size-dependent fashion (Supplementary Fig. 4), although intact core-shell MPs were not taken up, likely due to their much larger diameter. As tissue engineering generally requires protein delivery external to the cell [50], future work will aim to generate larger heparin MPs that cannot be taken up by cells, potentially enhancing the effectiveness of the heparin MP mediated protein delivery. Similar to core-shell MPs, heparin MPs also possess a size distribution, but various methods, including filtration and decreasing the surfactant concentration in the oil phase, could be employed in the future to reduce the formation of small MPs that might be internalized. Regardless, the results of this work highlight the ability of degradable core-shell MPs to temporally modulate the delivery of a protein-laden heparin core.
While several studies have shown the potential for heparin-based materials to sequester endogenous proteins [7–9, 51, 52], none of these have included a temporal control over representation. Previously, core-shell MPs have been used to selectively sequester proteins through a size-exclusive shell to concentrate biomarkers [18–20, 53], but few, if any, technologies can sequester, physically isolate, and then re-release protein to the external environment. As the core-shell MPs presented in this work has the potential to achieve this, it is possible that this technology can enhance temporal control over protein presentation in the cellular microenvironment for future tissue engineering applications.
5. Conclusions
In these experiments, heparin-PEG core-shell MPs were fabricated using a re-emulsification technique to encapsulate pre-formed heparin MPs within a degradable PEG-based shell, thus creating MPs with tunable amounts of encapsulated heparin. In pre-fabrication load studies with BMP-2, degradable core-shell MPs initiated enhanced ALP activity as compared to non-degradable core-shell MPs, indicating these MPs can be used to temporally modulate protein presentation to cells. In addition, post-fabrication loading studies demonstrated that core-shell MPs were able to sequester BMP-2 through the PEG shell and then re-present that protein to cells to initiate enhanced ALP activity. Thus, the goals of developing a dynamic core-shell MP technology that preserved protein bioactivity, delivered cargo in a temporally controlled manner with no burst release, and sequestered and re-delivered proteins were achieved in this system. In the future, because the post-fabrication loading technique allows fully fabricated core-shell MPs to sequester protein, it could be extended to sequestration and release of cell-secreted proteins. Overall, the multifunctional core-shell technology presented here has the potential to temporally modulate the presentation of growth factors in the local cellular microenvironment and is therefore a unique tool to explore the emerging area of cell-secreted protein manipulation for enhancement of tissue regeneration.
Supplementary Material
Statement of Significance.
Tissue repair requires temporally controlled presentation of potent proteins. Recently, biomaterial-mediated binding (sequestration) of cell-secreted proteins has emerged as a strategy to harness the regenerative potential of naturally produced proteins, but this strategy currently only allows immediate amplification and re-delivery of these signals. The multifunctional, dynamic core-shell heparin-PEG microparticles presented here overcome this limitation by sequestering proteins through a PEG-based shell onto a protein-protective heparin core, temporarily isolating bound proteins from the cellular microenvironment, and re-delivering proteins only after degradation of the PEG-based shell. Thus, these core-shell microparticles have potential to be a novel tool to harness and isolate proteins produced in the cellular environment and then control when proteins are re-introduced for the most effective tissue regeneration and repair.
Acknowledgments
We would like to acknowledge the Bellamkonda lab, especially Dr. Balakrishna Pai and Dr. Tarun Saxena, for their assistance with the extrusion device. We also wish to acknowledge the core facilities at the Parker H. Petit Institute for Bioengineering and Bioscience at the Georgia Institute of Technology for the use of their shared equipment, services, and expertise, especially Andrew Shaw for his help with confocal microscopy.
Funding Sources
This study was supported with funding from the National Science Foundation (NSF) Graduate Research Fellowship (DGE-1148903) to TER, the Georgia Tech Petit Scholar Program to BDP, NSF (DMR 1207045), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR063692. This content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 2.Lindahl U, Li J. Int. Rev. Cell Mol. Biol. 1st. Elsevier Inc; 2009. Chapter 3 Interactions Between Heparan Sulfate and Proteins—Design and Functional Implications; pp. 105–159. [DOI] [PubMed] [Google Scholar]
- 3.Lee KY, Yuk SH. Polymeric protein delivery systems. Prog. Polym. Sci. 2007;32:669–697. [Google Scholar]
- 4.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 5.a Kinney M, McDevitt TC. Emerging strategies for spatiotemporal control of stem cell fate and morphogenesis. Trends Biotechnol. 2013;31:78–84. doi: 10.1016/j.tibtech.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Impellitteri NA, Toepke MW, Lan Levengood SK, Murphy WL. Specific VEGF sequestering and release using peptide-functionalized hydrogel microspheres. Biomaterials. 2012;33:3475–3484. doi: 10.1016/j.biomaterials.2012.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah RN, a Shah N, Del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc. Natl. Acad. Sci. U. S. A. 2010;107:3293–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seto SP, Casas ME, Temenoff JS. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 2012;347:589–601. doi: 10.1007/s00441-011-1265-8. [DOI] [PubMed] [Google Scholar]
- 9.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 10.Lin C-C, Metters AT, Anseth KS. Functional PEG-peptide hydrogels to modulate local inflammation induced by the pro-inflammatory cytokine TNFalpha. Biomaterials. 2009;30:4907–4914. doi: 10.1016/j.biomaterials.2009.05.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freudenberg U, Zieris A, Chwalek K, Tsurkan MV, Maitz MF, Atallah P, Levental KR, Eming Sa, Werner C. Heparin desulfation modulates VEGF release and angiogenesis in diabetic wounds. J. Control. Release. 2015;220:79–88. doi: 10.1016/j.jconrel.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 12.Peng Y, Tellier LE, Temenoff JS. Heparin-based hydrogels with tunable sulfation & degradation for anti-inflammatory small molecule delivery. Biomater. Sci. 2016;4:1371–1380. doi: 10.1039/c6bm00455e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tellier LE, Miller T, McDevitt TC, Temenoff JS. Hydrolysis and sulfation pattern effects on release of bioactive bone morphogenetic protein-2 from heparin-based microparticles. J. Mater. Chem. B. 2015;3:8001–8009. doi: 10.1039/C5TB00933B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi DH, Park CH, Kim IH, Chun HJ, Park K, Han DK. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J. Control. Release. 2010;147:193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 15.Wu H, Liao C, Jiao Q, Wang Z, Cheng W, Wan Y. Fabrication of core-shell microspheres using alginate and chitosan-polycaprolactone for controlled release of vascular endothelial growth factor. React. Funct. Polym. 2012;72:427–437. [Google Scholar]
- 16.Wen Y, Gallego MR, Nielsen LF, Jorgensen L, Møller EH, Nielsen HM. Design and characterization of core-shell mPEG-PLGA composite microparticles for development of cell-scaffold constructs. Eur. J. Pharm. Biopharm. 2013;85:87–98. doi: 10.1016/j.ejpb.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 17.Wu J, Kong T, Yeung KWK, Shum HC, Cheung KMC, Wang L, To MKT. Fabrication and characterization of monodisperse PLGA-alginate core-shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013;9:7410–7419. doi: 10.1016/j.actbio.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Tamburro D, Fredolini C, Espina V, a Douglas T, Ranganathan A, Ilag L, Zhou W, Russo P, Espina BH, Muto G, Petricoin EF, a Liotta L, Luchini A. Multifunctional core-shell nanoparticles: discovery of previously invisible biomarkers. J. Am. Chem. Soc. 2011;133:19178–19188. doi: 10.1021/ja207515j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longo C, Gambara G, Espina V, Luchini A, Bishop B, Patanarut AS, Petricoin EF, Beretti F, Ferrari B, Garaci E, De Pol A, Pellacani G, a Liotta L. A novel biomarker harvesting nanotechnology identifies Bak as a candidate melanoma biomarker in serum. Exp. Dermatol. 2011;20:29–34. doi: 10.1111/j.1600-0625.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longo C, Patanarut A, George T, Bishop B, Zhou W, Fredolini C, Ross MM, Espina V, Pellacani G, Petricoin EF, a Liotta L, Luchini A. Core-shell hydrogel particles harvest, concentrate and preserve labile low abundance biomarkers. PLoS One. 2009;4:e4763. doi: 10.1371/journal.pone.0004763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.She Z, Wang C, Li J, Sukhorukov GB, Antipina MN. Encapsulation of basic fibroblast growth factor by polyelectrolyte multilayer microcapsules and its controlled release for enhancing cell proliferation. Biomacromolecules. 2012;13:2174–2180. doi: 10.1021/bm3005879. [DOI] [PubMed] [Google Scholar]
- 22.Choi DH, Subbiah R, Kim IH, Han DK, Park K. Dual growth factor delivery using biocompatible core-shell microcapsules for angiogenesis. Small. 2013;9:3468–3476. doi: 10.1002/smll.201300427. [DOI] [PubMed] [Google Scholar]
- 23.Lin C-C, Metters AT. Hydrogels in controlled release formulations: network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006;58:1379–1408. doi: 10.1016/j.addr.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 24.Liang Y, Kiick KL. Heparin-functionalized polymeric biomaterials in tissue engineering and drug delivery applications. Acta Biomater. 2014;10:1588–1600. doi: 10.1016/j.actbio.2013.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hettiaratchi MH, Miller T, Temenoff JS, Guldberg RE, McDevitt TC. Heparin microparticle effects on presentation and bioactivity of bone morphogenetic protein-2. Biomaterials. 2014;35:7228–7238. doi: 10.1016/j.biomaterials.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Billings PC, Pacifici M, Interactions of signaling proteins. growth factors and other proteins with heparan sulfate: mechanisms and mysteries. Connect. Tissue Res. 2015;56:272–280. doi: 10.3109/03008207.2015.1045066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kan M, Wang F, Xu J, Crabb JW, Hou J, Mckeehan WL. An Essential Heparin-Binding Domain in the Fibroblast Growth Factor Receptor Kinase. Science (80-.) 1993;259:1918–1291. doi: 10.1126/science.8456318. [DOI] [PubMed] [Google Scholar]
- 28.Seto SP, Miller T, Temenoff JS. Effect of selective heparin desulfation on preservation of bone morphogenetic protein-2 bioactivity after thermal stress. Bioconjug. Chem. 2015;26:286–293. doi: 10.1021/bc500565x. [DOI] [PubMed] [Google Scholar]
- 29.Weber LM, Lopez CG, Anseth KS. Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function. J. Biomed. Mater. Res. Part A. 2009;90A:720–729. doi: 10.1002/jbm.a.32134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ifkovits JL, a Burdick J. Review: photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 2007;13:2369–2385. doi: 10.1089/ten.2007.0093. [DOI] [PubMed] [Google Scholar]
- 31.van de Wetering P, Metters AT, Schoenmakers RG, a Hubbell J. Poly(ethylene glycol) hydrogels formed by conjugate addition with controllable swelling, degradation, and release of pharmaceutically active proteins. J. Control. Release. 2005;102:619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- 32.Hahn MS, Taite LJ, Moon JJ, Rowland MC, Ruffino KA, West JL. Photolithographic patterning of polyethylene glycol hydrogels. Biomaterials. 2006;27:2519–2524. doi: 10.1016/j.biomaterials.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 33.Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-sehara A, Suda T. Bone Morphogenic Protein-2 Converts the Differentiation Pathway of C2C12 Myoblasts into the Osteoblast Lineage. J. Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mitragotri S, Lahann J. Physical approaches to biomaterial design. Nat. Mater. 2009;8:15–23. doi: 10.1038/nmat2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dendukuri D, Doyle PS. The Synthesis and Assembly of Polymeric Microparticles Using Microfluidics. Adv. Mater. 2009;21:4071–4086. [Google Scholar]
- 36.Cha C, Jeong JH, Kong H. Poly(ethylene glycol)-poly(lactic-co-glycolic acid) core- shell microspheres with enhanced controllability of drug encapsulation and release rate. J. Biomater. Sci. Polym. Ed. 2015;26:828–840. doi: 10.1080/09205063.2015.1058575. [DOI] [PubMed] [Google Scholar]
- 37.Kim KK, Pack DW. Microspheres for Drug Delivery. In: Ferrari M, editor. BioMEMS Biomed. Nanotechnol. US, Boston, MA: Springer; 2006. pp. 19–50. [Google Scholar]
- 38.Göpferich A, Alonso M, Langer R. Development and characterization of microencapsulated microspheres. Pharm. Res. 1994;11 doi: 10.1023/a:1018901619230. [DOI] [PubMed] [Google Scholar]
- 39.Siltanen C, Yaghoobi M, Haque A, You J, Lowen J, Soleimani M, Revzin A. Microfluidic fabrication of bioactive microgels for rapid formation and enhanced differentiation of stem cell spheroids. Acta Biomater. 2016;34:1–8. doi: 10.1016/j.actbio.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deveza L, Ashoken J, Castaneda G, Tong X, Keeney M, Han L-H, Yang F. Microfluidic Synthesis of Biodegradable Polyethylene-Glycol Microspheres for Controlled Delivery of Proteins and DNA Nanoparticles. ACS Biomater. Sci. Eng. 2015;1:157–165. doi: 10.1021/ab500051v. [DOI] [PubMed] [Google Scholar]
- 41.Headen DM, Aubry G, Lu H, García AJ. Microfluidic-based generation of size-controlled, biofunctionalized synthetic polymer microgels for cell encapsulation. Adv. Mater. 2014;26:3003–3008. doi: 10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parlato M, Johnson A, Hudalla Ga, Murphy WL. Adaptable Poly(ethylene glycol) Microspheres Capable of Mixed-mode Degradation. Acta Biomater. 2013;9:9270–9280. doi: 10.1016/j.actbio.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131:6009–6021. doi: 10.1242/dev.01522. [DOI] [PubMed] [Google Scholar]
- 44.Lim MPA, Lee WL, Widjaja E, Loo SCJ. One-step fabrication of core-shell structured alginate-PLGA/PLLA microparticles as a novel drug delivery system for water soluble drugs. Biomater. Sci. 2013;1:486. doi: 10.1039/c3bm00175j. [DOI] [PubMed] [Google Scholar]
- 45.Somoza RA, Welter JF, Correa D, Caplan AI. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014;20:596–608. doi: 10.1089/ten.teb.2013.0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ten Broek RW, Grefte S, Von Den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J. Cell. Physiol. 2010;224:7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 47. [accessed October 8, 2016];R&D Systems Incorporated, R&D Systems. https://www.rndsystems.com/
- 48.Bell CL, Peppas Na. Water, solute and protein diffusion in physiologically responsive hydrogels of poly(methacrylic acid-g-ethylene glycol) Biomaterials. 1996;17:1203–1218. doi: 10.1016/0142-9612(96)84941-6. [DOI] [PubMed] [Google Scholar]
- 49.Peppas Na, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical Foundations and Structural Design of Hydrogels in Medicine and Biology. Annu. Rev. Biomed. Eng. 2000;2:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 50.Ye M, Kim S, Park K. Issues in long-term protein delivery using biodegradable microparticles. J. Control. Release. 2010;146:241–260. doi: 10.1016/j.jconrel.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 51.Cushing MC, Liao J-T, Jaeggli MP, Anseth KS. Material-based regulation of the myofibroblast phenotype. Biomaterials. 2007;28:3378–3387. doi: 10.1016/j.biomaterials.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Hettiaratchi MH, Guldberg RE, McDevitt TC. Biomaterial Strategies for Controlling Stem Cell Fate Via Morphogen Sequestration. J. Mater. Chem. B. 2016;4:3464–3481. doi: 10.1039/c5tb02575c. [DOI] [PubMed] [Google Scholar]
- 53.Basinska T. Hydrophilic Core-Shell Microspheres: A Suitable Support for Controlled Attachment of Proteins and Biomedical Diagnostics. Macromol. Biosci. 2005;5:1145–1168. doi: 10.1002/mabi.200500138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.