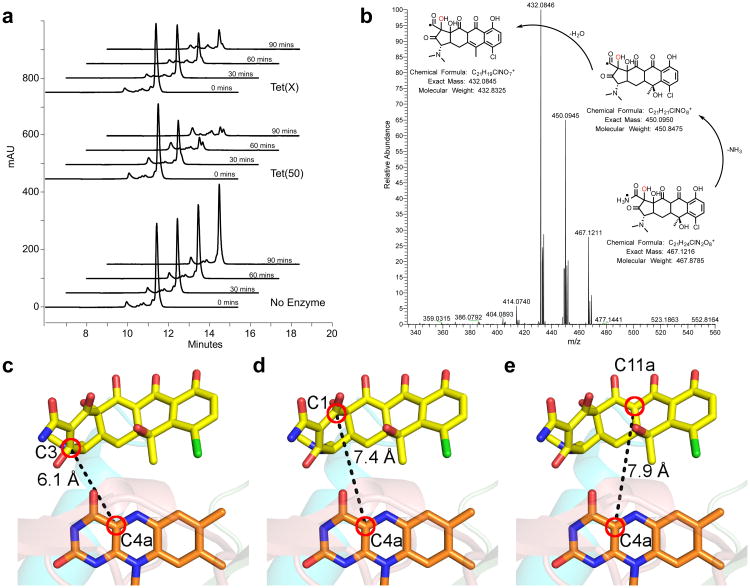
Figure 4. Chlortetracycline is degraded by tetracycline destructases despite the unusual binding mode.
(a) HPLC chromatograms show the time and enzyme dependent consumption of chlortetracycline. (b) High-resolution MS-MS analysis of the tetracycline destructase reaction with chlortetracycline supports clean conversion to the m/z 467 oxidation product. MS-MS spectrum of the m/z 467 ion from the Tet(55) reaction with proposed fragmentation pathway. c-e The closest reactive carbons to C4a of the FAD cofactor are C3 (c) and C1 (d) of the chlortetracycline A ring, both of which are closer than C11a (e), the hydroxylation site observed in Tet(X) mediated chlortetracycline degradation.