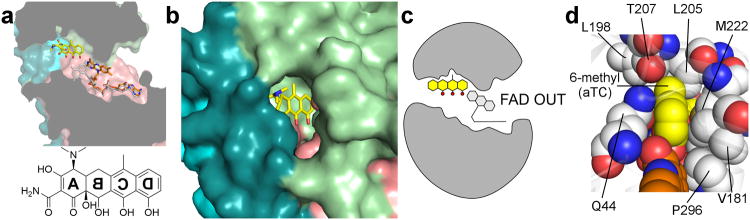
Figure 5. Anhydrotetracycline binds to the active site of Tet(50), trapping FAD in the unproductive OUT conformation.
(a) Anhydrotetracycline binds the active site of Tet(50) and traps the FAD cofactor in the unproductive OUT conformation (orange) in monomer B. The IN conformation of FAD from monomer A is superimposed in grey for comparison, and sterically clashes with the D-ring hydroxyl of anhydrotetracycline. (b) Surface representation of Tet(50)+anhydrotetracycline reveals that the substrate-loading channel remains open, which corresponds to FAD locked in the OUT conformation. (c) In Tet(50)+anhydrotetracycline monomer B, FAD is OUT, the loop is open, and anhydrotetracycline is bound (not shown: in monomer A, FAD is IN, the loop is closed, no anhydrotetracycline is bound). (d) Residue Thr-207 in Tet(50) makes van der Waals interactions with the planar 6-methyl group of anhydrotetracycline (aTC) (yellow) in the bound orientation, but would sterically clash with the 6-methyl and 6-hydroxyl groups that branch from the C ring of tetracycline or chlortetracycline if bound in a flipped orientation.