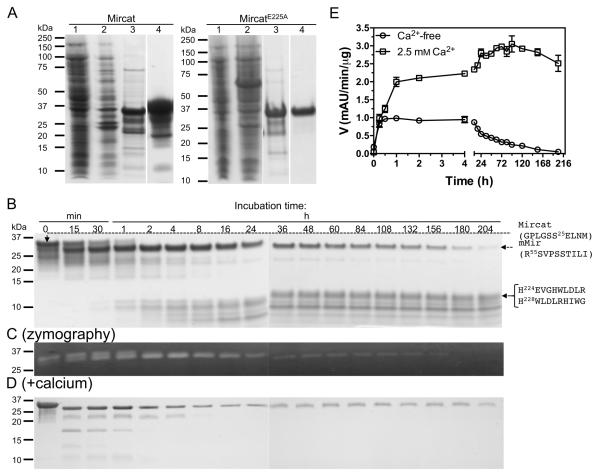
Figure 3.
Expression, purification, and the effect of calcium on autoprocessing of mirolysin without C-terminal extension (CTE). A) Catalytic domain preceded by the prodomain of mirolysin and its active site mutein (Mircat and MircatE225A, respectively) were obtained as recombinant proteins and purified by affinity chromatography on glutathione-Sepharose with simultaneous removal of the GST tag by PreScission cleavage followed by size exclusion chromatography (SEC) and ion exchange chromatography (MircatE225A only). Lanes 1 and 2, E. coli extracts before and 8 h after induction of protein expression with IPTG, respectively; lanes 3 and 4, proteins after purification by affinity chromatography and SEC followed by ion exchange chromatography for MircatE225A only, respectively. B-E) Mircat was incubated at 0.5 mg/ml in 50 mM Tris, 0.02% NaN3 pH 8.0, alone (B) or supplemented with 2.5 mM CaCl2 (D). At specific time points, aliquots were taken and analyzed by SDS-PAGE (B, D) followed by zymography (C, for incubation in calcium-free buffer only) and measurement of residual activity against Azocoll as a substrate (E). N-terminal sequences determined by Edman degradation are shown on the right.