Abstract
Background/Objectives
To assess symptoms in older (age ≥65 years) intensive care unit (ICU) survivors and determine whether post-ICU frailty identifies those with the greatest palliative care needs.
Design
A prospective cohort study.
Setting
An urban tertiary-care hospital and community hospital.
Participants
One-hundred and twenty-five medical-ICU survivors of mechanical ventilation age ≥65 years.
Measurements
Baseline measurements of the Edmonton Symptom Assessment Scales (ESAS), categorized as mild (0–3), moderate (4–6), and severe (7–10), and the frailty phenotype were made during the week prior to hospital discharge. Functional recovery was defined as a return to a Katz Activities of Daily Living dependency count less than or equal to the pre-hospitalization dependency count within 3 months. In the last 29 participants, we made additional assessments of fatigue and ESAS at baseline and 1 month after discharge.
Results
Fatigue was the most prevalent moderate-to-severe symptom (74%), followed by dyspnea (53%), drowsiness (50%), poor appetite (47%), pain (45%), depression (42%), anxiety (36%), and nausea (17%). At 1 month follow-up, there were no significant differences in the proportions of participants with moderate-to-severe symptoms. Each increase in baseline ESAS fatigue severity category was associated with a 55% lower odds of functional recovery (OR 0.45, 95% CI 0.24–0.84), independent of age, sex, comorbidities, and critical illness severity. Compared to non-frail participants, frail participants had a higher median (IQR) baseline total ESAS symptom distress score (13 [9–22] versus 34 [23–44], p <0.001).
Conclusions
Older ICU survivors have a high burden of palliative care needs that persist at 1 month after discharge. Fatigue is the most prevalent symptom and may interfere with recovery. Post-ICU frailty may be both a useful palliative care consultation trigger and treatment target.
Keywords: palliative care, fatigue, frailty, critical care
INTRODUCTION
The largest group of intensive care unit (ICU) survivors with the highest proportion of adverse outcomes are older survivors of mechanical ventilation.1 Approximately 150,000 older adults survive an ICU admission with invasive mechanical ventilation annually in the United States, and this number will grow exponentially with the aging of our population.1–3 Two-thirds of older ICU survivors of mechanical ventilation require post-acute facility care and 30% die within 6 months.1
Previous palliative care interventions have focused on critically ill patients within the ICU.4–7 These studies have yielded mixed results and largely ignore the fact that most older adults survive the ICU and spend a week or more on the general ward prior to discharge.8 A comprehensive assessment of the palliative care needs of older ICU survivors has never been performed just prior to hospital discharge and during the early post-acute care period. An improved understanding of patients’ needs and preferences during this important care transition may contribute to the development of tailored approaches to post-ICU care for this rapidly growing and debilitated population of older adults.
The use of triggers for palliative care consultation, i.e. clinical criteria associated with a high symptom burden or poor outcomes, has been advocated in ICUs to ensure appropriate specialist involvement for patients at high risk of unmet palliative care needs.9, 10 While several studies have successfully utilized ICU triggers to improve palliative care,5, 7 no studies have yet identified post-ICU triggers for critical illness survivors. Frailty is a syndrome characterized by a generalized vulnerability to stressors resulting from an accumulation of physiologic deficits across multiple interrelated systems.11 We have shown that post-ICU frailty, measured by Fried’s frailty index during the post-critical acute care period, is associated with incident disability and high 6-month mortality in older ICU survivors.11, 12 We therefore undertook a prospective cohort study to assess the physical and psychological symptom distress and end-of-life care preferences of older ICU survivors of mechanical ventilation, and to assess whether post-ICU frailty identifies those with the greatest unmet palliative care needs just prior to hospital discharge.
METHODS
Study Design & Participants
We conducted a prospective cohort study at Columbia University Medical Center, a tertiary-care center, and the Allen Pavilion, a Columbia-affiliated New York City community hospital. Recruitment took place in two phases using the same eligibility criteria: a pilot cohort (n=22) was enrolled between February and August 2012, and the main cohort (n=103) was enrolled between May 2014 and February 2016. Eligible patients were ≥65 years old and admitted to the medical-ICU for acute respiratory failure requiring >24 hours of invasive or non-invasive (continuous or bi-level positive pressure support) mechanical ventilation. Exclusion criteria were use of extracorporeal life support, prior lung transplantation, current or previous neurological injury or neuromuscular disease with motor deficits, respiratory failure due to a primary neurologic diagnosis, dementia or altered mental status with inability to follow commands, not English or Spanish speaking, absence of a surrogate, in-hospital death, and planned discharge with hospice care. We excluded participants for whom we could not determine frailty status due to an incomplete Fried frailty assessment. The rationale for inclusion and exclusion criteria and details regarding informed consent are described in Supplementary Methods S1. The Columbia University Medical Center Institutional Review Board approved this study.
Measurements
Baseline measurements were made during the week prior to hospital discharge after participants were transferred from the ICU to the medical ward. Follow-up symptom assessments were made in-person or via telephone 1 month after hospital discharge. We measured symptoms at baseline with the Edmonton Symptom Assessment Scales (ESAS), assessing the severity of nine symptoms on 0–10 integer scales (pain, tiredness, nausea, depression, anxiety, drowsiness, poor appetite, dyspnea, and overall wellbeing).13 We assumed tiredness was a measure of fatigue, as has been done in prior studies.14, 15 As the ESAS survey methods direct, the surrogate estimated symptom severity when the participant could not. We categorized the severity of symptoms using the validated cut-offs of mild (0–3), moderate (4–6), and severe (7–10).16 We examined symptom burden as the physical symptom distress score (a sum of pain, fatigue, nausea, drowsiness, dyspnea, and poor appetite totaling 0–60); the emotional symptom distress score (a sum of anxiety and depression totaling 0–20); and, the total symptom distress score (a sum of the physical score, the emotional score, and wellbeing totaling 0–90).15
Interim analyses of the first 96 participants revealed that participants reported moderate-to-severe fatigue more frequently than any other ESAS symptom. Since fatigue can have multiple etiologies, we added questionnaires at baseline and 1 month to better characterize the potential causes of fatigue and the impact of fatigue on physical and social function. The added questionnaires included the Brief Fatigue Inventory (BFI), the patient health questionnaire-9 (PH-9Q), the Insomnia Severity Index (ISI)), and added ESAS assessments at 1 month. Supplementary Methods S1 contains further details about these questionnaires.
We assessed end-of-life care preferences by asking the participant or surrogate as appropriate: whether the participant has a preference for goals of care that focus on comfort over life-prolonging care, and whether the participant would desire cardiopulmonary resuscitation and mechanical ventilation if death were imminent.17 Do Not Resuscitate (DNR) status was obtained from the medical record.
We assessed Fried’s five frailty domains during the baseline assessment. We used the original criteria to determine the presence or absence of each component, and frailty was defined as having ≥3 of these components.11 We made minor modifications to address measurement challenges related to the population of older ICU survivors that we have published previously,12 and that are described in Supplementary Table S1.
We assessed sociodemographic variables, Charlson comorbidities, the Acute Physiology and Chronic Health Evaluation (APACHE) II score, duration and type of mechanical ventilation, length of stay, and palliative care consultations during the hospitalization. We screened for cognitive function at baseline with the Mini-Cog.18 We assessed disability as the number of dependencies in the Katz Activities of Daily Living (ADLs) recalled from 1 month prior to hospitalization using a validated method,19 at the baseline assessment, and at 3 month follow-up.20 Functional recovery was defined as a return to a Katz ADL dependency count less than or equal to the pre-hospitalization dependency count within 3 months.21 Decedents were categorized as not achieving functional recovery. We examined hemoglobin levels and medications given during the in-hospital post-critical acute care period for participants who underwent the additional fatigue assessments (see Supplementary Methods S1 for details).
Statistical Analyses
We compared categorical variables using Chi-square or Fischer exact tests, and compared continuous variables using unpaired t-tests or Wilcoxon rank sum tests. We compared changes in symptom scores between the baseline assessment and 1-month follow-up using the Wilcoxon signed rank test. Using previously validated minimally clinically important differences (MCIDs) for ESAS individual, emotional, physical, and total symptom distress scores,15, 22 we determined the proportion of participants who had improvement, no change, or worsening in their individual and composite ESAS symptom distress scores between baseline and 1 month. We assessed associations of composite ESAS symptom distress scores by tertiles of age. We report ESAS sum score medians (IQR) for each age-group tertile, and the p-value for interaction between frailty and age in a linear regression model with ESAS score as the dependent variable. We used the Cochran-Armitage test for trend to test associations between the proportions of participants with potential secondary causes of fatigue across categories of increasing ESAS fatigue severity. We used logistic regression to test the association between categories of ESAS fatigue severity at baseline and functional recovery at 3 months. A two-tailed p-value <0.05 was considered significant. We conducted an agglomerative hierarchical cluster analysis on the nine ESAS symptoms rated at baseline. Data were analyzed using Stata14.0 (Stata-Corp LP, College Station, TX) and RStudio 0.99.484 (R foundation, Vienna, Austria).
RESULTS
Participants
We screened 503 older medical ICU survivors; 339 were excluded based on pre-specified criteria, 26 were missed, 26 declined, and 9 were excluded for having incomplete frailty assessments (Supplementary Figure S1). The final study sample included 125 participants, of who 22 were pilot study participants whose screening and enrollment data have been published previously.12 A total of 41 participants (12 from the pilot cohort and 29 from the main cohort) completed the ESAS at baseline and 1 month. Twenty-nine participants from the main cohort completed questionnaires related to fatigue at baseline and 1 month.
Participants had a median (IQR) age of 74 (68–81) years and 107 (86%) were frail. Compared to non-frail participants, frail participants were more often admitted from skilled-care facilities and had a higher comorbidity burden. While frail and non-frail participants had a similar severity of critical illness based on the APACHE II score, frail participants more often required invasive mechanical ventilation and had a longer length of stay. Frail participants had a higher prevalence of cognitive impairment compared to non-frail participants (36% vs. 6%). Only 7 participants (6%) received a palliative care consult prior to hospital discharge, all of who were frail. At 3 months after hospital discharge, frail participants had a higher median (IQR) number of ADL dependences compared to non-frail participants (1 (0–3) versus 0 (0-0), and were more likely to have died (23% versus 0%) (Table 1).
Table 1.
Participant Characteristics by Frailty Status
| Characteristics of Participants | No. with data |
All (n=125) |
Frail (n=107) |
Non-Frail (n=18) |
|---|---|---|---|---|
| Female, n (%) | 125 | 64 (51%) | 58 (54%) | 6 (33%) |
| Age, median (IQRa) | 125 | 74 (68 – 81) | 76 (68 – 81) | 70 (67 – 74) |
| Race/Ethnicity | 125 | |||
| Non-Hispanic White, n (%) | 42 (34%) | 33 (31%) | 9 (50%) | |
| Non-Hispanic Black, n (%) | 17 (14%) | 17 (16%) | 0 (0%) | |
| Hispanic, n (%) | 64 (51%) | 55 (51%) | 9 (50%) | |
| Other, n (%) | 2 (2%) | 2 (2%) | 0 (0%) | |
| Health Insurance | 125 | |||
| Medicare Only, n (%) | 20 (16%) | 18 (17%) | 2 (11%) | |
| Medicaid, n (%) | 73 (58%) | 65 (61%) | 8 (44%) | |
| Private, n (%) | 32 (26%) | 24 (22%) | 8 (44%) | |
| Highest Level Education | 120 | |||
| ≤ High School Degree, n (%) | 80 (67%) | 69 (66%) | 11 (69%) | |
| ≥ College Degree, n (%) | 40 (33%) | 35 (34%) | 5 (31%) | |
| Residence Prior to Hospitalization | 125 | |||
| Home, n (%) | 95 (76%) | 78 (73%) | 17 (94%) | |
| Skilled-Care Facilityb, n (%) | 30 (24%) | 29 (27%) | 1 (6%) | |
| Pre-Admission Katz ADL Index, median (IQR) |
124 | 0 (0 – 3) | 1 (0 – 3) | 0 (0 – 0) |
| Charlson Comorbidity Index, median (IQR) |
125 | 2 (1 – 4) | 2 (1 – 4) | 1 (0 – 1) |
| Metastatic or Liquid Tumor, n (%) | 125 | 10 (8%) | 9 (8%) | 1 (6%) |
| APACHEc II Score, median (IQR) | 125 | 31 (26 – 37) | 32 (26 – 37) | 30 (24 – 38) |
| Type of Respiratory Support | 125 | |||
| Mechanical Ventilation, n (%) | 100 (80%) | 87 (81%) | 13 (72%) | |
| Noninvasive Ventilation Only, n (%) | 25 (20%) | 20 (19%) | 5 (31%) | |
| ICUd days, median (IQR) | 125 | 4 (3 – 8) | 5 (3 – 8) | 2 (2 – 4) |
| Post-ICU Ward Days Before Discharge, median (IQR) |
125 | 7 (4 – 13) | 7 (4 – 15) | 4 (2 – 7) |
| Total Hospital Days, median (IQR) | 125 | 12 (8 – 22) | 13 (9 – 26) | 8 (3 – 12) |
| Cognitive Impairmente, n (%) | 120 | 35 (31%) | 34 (36%) | 1 (6%) |
| Deliriumf, n (%) | 120 | 7 (6%) | 7 (7%) | 0 (0%) |
| Palliative Care Consult, n (%) | 125 | 7 (6%) | 7 (7%) | 0 (0%) |
| Disposition at Hospital Discharge | 125 | |||
| Home, n (%) | 63 (50%) | 46 (43%) | 17 (94%) | |
| Skilled-Care Facility, n (%) | 62 (50%) | 61 (57%) | 17 (6%) | |
| 3-month Mortality, n (%) | 110 | 23 (18%) | 23 (21%) | 0 (0%) |
| 3-month Katz ADL Index, median (IQR)g |
74 | 1 (0 – 3) | 1 (0 – 3) | 0 (0 – 0) |
Interquartile range;
Skilled care facilities included acute or sub-acute rehabilitation facilities, residential nursing homes, or long-term acute care facilities;
Acute Physiology And Chronic Health Evaluation;
Intensive Care Unit;
Mini-Cog screening;
Confusion Assessment Method-ICU screening;
Does not include 22 pilot study participants who did not have these measurements; 17 participants died before 3-month follow-up, 9 participants are awaiting 3-month follow-up, and 3 participants could not be reached for 3-month follow-up
Symptoms and Frailty During the Week Prior to Hospital Discharge
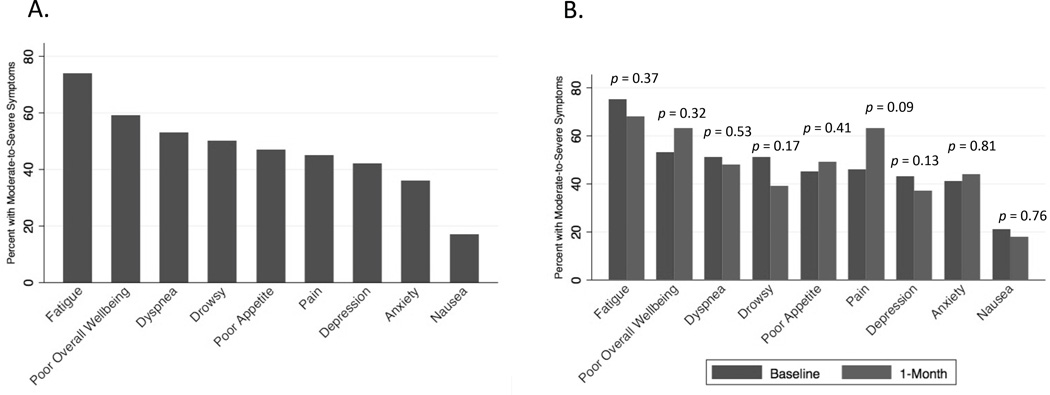
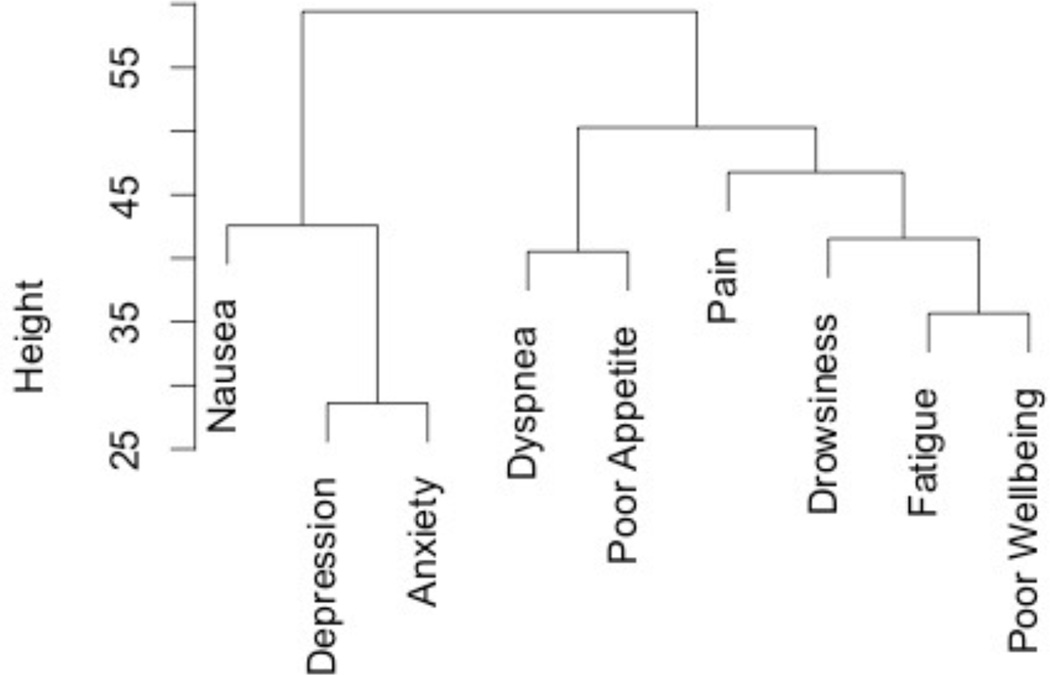
Fatigue was the most prevalent moderate-to-severe symptom (74%), followed by dyspnea (53%), drowsiness (50%), poor appetite (47%), pain (45%), depression (42%), anxiety (36%), and nausea (17%) (Figure 1A). The dendrogram suggests that there are two distinct symptom clusters (Figure 2). In one cluster, fatigue clustered most closely with worse overall wellbeing and the physical symptoms of drowsiness, pain, dyspnea, and appetite. In the other cluster, depression and anxiety clustered closely together, followed by nausea.
Figure 1.
(A) Percent of participants with moderate-to-severe ESAS Symptoms at Baseline (n = 125) (B) Percent of participants with moderate-to-severe ESAS symptoms at baseline and 1 month (n = 41). Symptom severity is defined as moderate-to-severe if the severity score is ≥4.
Figure 2.
A cluster dendrogram of ESAS symptoms at baseline illustrating the history of merges resulting in the depicted clusters, with the vertical height between symptoms defined by the Euclidian distance, and symptom cluster node distance defined by Ward’s minimum variance.
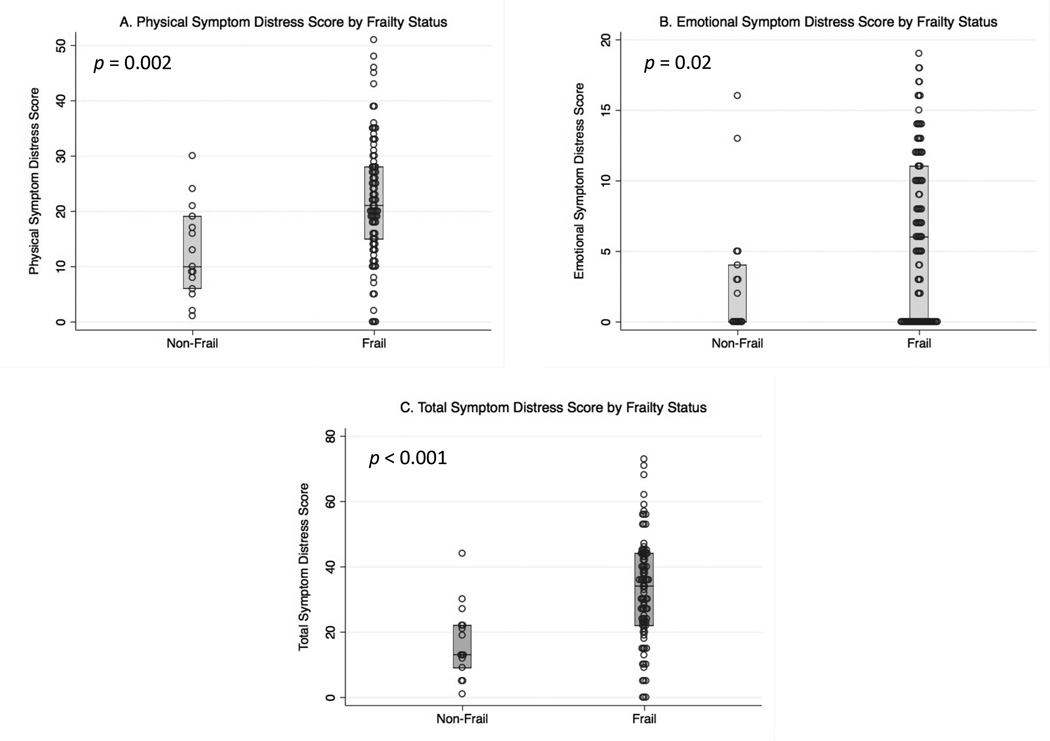
Compared to non-frail participants, frail participants had significantly higher emotional, physical, and total ESAS symptom distress scores (Figure 3). The statistically significant differences in the median emotional symptom scores (6 points), physical symptom scores (12 points), and total symptom scores (21 points) between frail and non-frail participants exceed the MCIDs of 3 points, 2 points, and 3 points, respectively.15 Compared to non-frail participants, frail participants reported significantly higher levels of fatigue, drowsiness, anxiety, and worse overall wellbeing (Supplementary Table S2). Among frail participants, neither individual nor sum scores of ESAS symptoms differed significantly across frailty scores 3, 4, and 5 (Supplementary Table S3).
Figure 3.
(A) Physical, (B) emotional, and (C) total ESAS symptom distress scores by frailty status. The physical symptom distress score is the composite score for ESAS symptoms of tiredness, drowsiness, dyspnea, nausea, pain, and poor appetite. The median (IQR) for the physical symptom distress score was 9 (5–17) for non-frail participants, and 21 (15 – 28) for frail participants (p <0.001). (B) The emotional symptom distress score is the composite score for ESAS symptoms of depression and anxiety. The median (IQR) for the emotional symptom distress score was 0 (0–4) for non-frail participants and 6 (0–11) for frail participants (p = 0.02). (C) The total symptom distress score is the composite score for all nine ESAS symptoms (those aforementioned and overall wellbeing). The median (IQR) for the total ESAS symptom distress score was 13 (8–22) for non-frail participants and 34 (23 – 44) for frail participants (p <0.001).
There was no statistically significant difference in composite ESAS symptom distress scores by tertiles of age (Supplementary Table S4), or by comparing the 7 participants who received a palliative care consultation to those who did not (Supplementary Table S5).
End-of-Life Care Preferences and Frailty During the Week Prior to Hospital Discharge
While 95 (80%) participants reported a desire for comfort-oriented end-of-life care and 74 (62%) were willing to forgo cardiopulmonary resuscitation, only 24 (19%) had a DNR status. Compared to non-frail participants, frail participants were not significantly more likely to prefer comfort-oriented end-of-life care (71% versus 81%, p=0.31) or have a DNR status (11% versus 21%, p=0.35). Among the 7 participants who received a palliative care consultation during the hospitalization, 6 had documentation of discussions about advanced care planning, 3 changed to a DNR status after the consultation, and 1 changed her hospital discharge plan from skilled-care to hospice after study enrollment.
Changes in Symptoms at 1 Month
There were no statistically significant differences in the proportions of participants reporting moderate-to-severe symptoms between baseline and 1 month (Figure 1B). The median ESAS emotional, physical, and total symptom distress scores did not differ significantly between baseline and 1 month either (Supplementary Table S4). Categorizing changes in ESAS symptom distress scores as improved, no change, and worsened based on MCIDs revealed that ESAS emotional and physical symptom distress scores improved for 33% and 44%, did not change for 45% and 17%, and worsened for 23% and 39%, respectively (Supplementary Table S5).
Fatigue Analyses
Among the 29 participants who completed the BFI, 74% and 59% felt unusually fatigued at baseline and at 1 month, respectively (p=0.28). At baseline, 62% and 66% reported that fatigue interfered with general activity and walking (scores >0 on 0–10 integer scales), respectively. At 1-month follow-up, 55% and 66% reported that fatigue interfered with general activity and walking, respectively (Supplementary Table S6). At baseline, 71% and 51% of those with moderate or severe fatigue had insomnia based on the ISI, whereas no participants with mild fatigue reported insomnia (p-for-trend = 0.06) (Supplementary Table S7). Depression was highly prevalent (94% based on the PHQ-9 criteria), and did not change across categories of increasing ESAS fatigue severity (p-for-trend=0.88) (Supplementary Table S7).
Among all 125 study participants, those reporting severe fatigue at baseline tended to have lower median (IQR) hemoglobin levels (9.6 (8.8–11.0 mg/dL) compared to those reporting mild fatigue (10.9 (9.1–11.9) mg/dl) (p-for-trend=0.07). The proportion of participants on medications at baseline with a potential side effect of fatigue did not change across categories of increasing ESAS fatigue severity (all p-for-trend >0.05) (Supplementary Table S7). Each increase in baseline ESAS fatigue severity category was associated with a 55% lower odds of functional recovery (OR 0.45 95% CI 0.24–0.84), independent of age, sex, comorbidities, and critical illness severity (Supplementary Table S8).
DISCUSSION
We have shown that ICU survivors of mechanical ventilation older than 65 years have a high burden of unmet palliative care needs just prior to hospital discharge, which persist or worsen for a majority during the month after hospital discharge. While prior critical care outcomes studies have focused on physical disability,23 cognitive impairment,24 and psychiatric illnesses,25 our study is the first to report a comprehensive symptom assessment across the post-ICU to post-acute care transition. We found that fatigue is the most common moderate-to-severe symptom in older ICU survivors, and that it may interfere with functional recovery. Our findings suggest that palliative care interventions are needed not only in the ICU but also during the post-ICU acute care period, and that the post-ICU frailty phenotype may be a useful palliative care trigger to study further since it identifies those with the greatest symptom burden.
There are several reasons why older ICU survivors may have unmet palliative care needs at hospital discharge. Distracted by the fact that patients survived a potentially life-threatening illness, providers may focus on the treatment of medical conditions and presume that symptoms resolve as patients recover. Many participants in our study had cognitive dysfunction on mini-COG screening, and these patients may have difficulty communicating their symptoms.26 Study participants may not have received a palliative care consultation because most of them do not have advanced cancer, dementia, or heart failure, the most common diagnoses for which palliative care is consulted and for which hospice services are received.27–29 It is for this very reason that frailty might be considered a trigger for palliative care consult, as it is associated with uncontrolled symptoms and may identify a patient population that is not traditionally considered in need of palliative care.
Our cross-sectional analysis does not permit an evaluation of the causal direction of the association between the frailty phenotype and symptom burden, but there are several reasons to believe that the pathways could be bidirectional. Persistent pain may perpetuate frailty by impairing mobility, leading to decreased nutritional intake, or causing depression and feelings of exhaustion.30 Dyspnea may lead to avoidance of physical activity, accelerating frailty due to deconditioning.31, 32 Alternatively, frailty may cause dyspnea. In community-dwelling older adults, reduced lower extremity proximal muscle function is independently associated with moderate to severe dyspnea,33 and slower gait speed is independently associated with worse respiratory muscle function.34 Frail older adults have been found to have deregulation of grehlin and cholecystokinin that may cause loss of appetite, and that in turn could potentiate muscle wasting and fatigue.35 The frailty phenotype is associated with depression and anxiety,36 and frailty and depression interact resulting in an increased risk of death in older adults.37 Given these associations, treating symptoms in frail older ICU survivors may be both palliative and potentially therapeutic, especially if symptoms are limiting rehabilitation or nutritional intake.
We found a high prevalence of discordance between reported end-of-life care preferences and documented code status in older ICU survivors just prior to hospital discharge. Patients and surrogates are typically asked about code status upon arrival to the hospital and during the ICU admission.38 Our results suggest that targeting the post-ICU pre-hospital discharge period for advance care planning discussions may allow patients the opportunity to reconsider their preferences based on actual experiences receiving life-sustaining therapies in the ICU.12
Fatigue is one of the most common, underreported, and undertreated symptoms in advanced cancer, AIDS, heart disease, and chronic obstructive pulmonary disease (COPD).39,40 In community-dwelling older adults, fatigue is independently associated with frailty,41 worse physical function,42 and is the leading reason for restricted activity.43 Our study is the first to find that higher levels of fatigue are independently associated with lower odds of functional recovery in older survivors of critical illness. Studies of older adults with COPD have shown that fatigue is independently associated with decreased physical activity,44 but COPD patients still achieve clinically significant improvements in fatigue and function with pulmonary rehabilitation.45 Accordingly, fatigued older ICU survivors might still benefit from rehabilitation. Still, fatigue is one of the most difficult symptoms to treat given its multifactorial nature and inconsistent response to pharmacologic therapy.46 These complexities underscore the importance of referring fatigued older ICU survivors to specialty-level palliative care.
Our finding that fatigue clusters most closely with drowsiness, pain, dyspnea, and loss of appetite, and is associated with insomnia in older ICU survivors, suggests that we should treat pain aiming to avoid opioid-induced drowsiness, assess and optimize respiratory function, promote adequate nutritional intake, and treat insomnia with environmental or pharmacologic interventions. Future research should further examine the relationships between fatigue, physical activity, and functional recovery in older ICU survivors, as well as evaluate therapeutic options for treating fatigue. For example, stimulants such as methylphenidate have been found to treat multifactorial fatigue in cancer patients,47 and have been shown to be safe for use in older adults with depression.48
Our study has several limitations. The ESAS only measures a subset of possible symptoms experienced by older ICU survivors. While the frailty phenotype identified ICU survivors with the highest burden of symptoms, 86% were frail using Fried’s original cutoffs that were derived using community-dwelling older adults. Rescaling frailty component cutoffs for the population of older ICU survivors may achieve greater differentiation of degrees of symptom burden and severity by frailty status. Several participants demonstrated cognitive dysfunction at enrollment, and the relationships between physical frailty, cognitive function, and outcomes need to be studied further. We began ESAS and detailed fatigue assessments at 1 month after hospital discharge only after a majority of participants had already enrolled. We had too few participants to examine ESAS symptom severity trajectories by frailty status. Future studies should track symptoms over longer periods of time and evaluate associations with frailty status. Study participants were treated at a tertiary-care center and community hospital in New York City. Future studies of larger cohorts of older ICU survivors from different geographic regions are needed to validate our results.
In conclusion, older ICU survivors have a high burden of unmet palliative care needs that persist at 1 month after discharge. Our findings support a paradigm expansion of palliative care interventions for the critically ill from early interventions in the ICU to in-hospital post-critical acute care interventions focused on advance care planning and treating symptoms in frail older ICU survivors with the goal of improving critical illness survivorship.
Supplementary Material
Table 2.
End-of-Life Care Preferences and Code Status by Frailty Score Categories
| End-of-Life Care Preference | All | Frail | Non-Frail | p |
|---|---|---|---|---|
| Preference for or consideration of comfort-oriented end-of-life care, n (%) |
95 (80%) | 83 (81%) | 12 (71%) | 0.31 |
| Would not want chest compressions or breathing machine, or unsure, n (%) |
74 (62%) | 62 (61%) | 12 (71%) | 0.44 |
| DNRa, n (%) | 24 (19%) | 22 (21%) | 2 (11%) | 0.35 |
Do Not Resuscitate
Acknowledgments
Sponsor’s role: The National Institutes of Health had no role in the design of the study, collection and analysis of the data, or in the preparation of the manuscript. LRP and MRB greatly appreciate Louise Callahan’s support of this research in honor of her late husband Noel Byrne.
Funding Sources: UL1 TR000040 (MRB), K23 AG045560 (MRB), AG 022846 Pilot Core (MRB), Columbia University Aging Center Faculty Research Fellowships (MRB), NIA and AFAR Medical Student Training in Aging Research (LRP), Columbia University College of Physicians and Surgeons Dean’s Research Fellowship (LRP), R01 HL114626 (DJL).
Footnotes
Conflicts of Interests: The authors declare no personal or financial conflicts of interest.
Author contributions: Study concept and design: MRB, NG, MSM, DJL. Acquisition of participants and/or data: MRB, LRP, WG. Data analysis and interpretation of data: LRP, MRB Preparation of Manuscript: LRP, MRB.
Supplementary Material:
1. Supplementary Methods S1 Rationale for Inclusion and Exclusion Criteria
2. Supplementary Tables and Figures
Supplementary Table S1. Frailty Criteria
Supplementary Table S2. Individual Symptom ESAS Scores by Frailty Status at Baseline
Supplementary Table S3. ESAS Symptom Scores by Frailty Score Among Frail Participants
Supplementary Table S4. Associations Between ESAS Composite Symptom Distress Scores and Age Categorized by Tertile
Supplementary Table S5. Associations Between ESAS Composite Symptom Distress Scores and Receipt of Palliative Care Consult
Supplementary Table S6. Median ESAS Composite Symptom Distress Scores at Baseline and 1-Month
Supplementary Table S7. Change in Individual ESAS Symptoms and Composite ESAS Symptom Distress Scores (Improved, Unchanged, Worsened) Between Baseline and 1-Month Based on Validated Minimal Clinically Important Differences
Supplementary Table S8. Brief Fatigue Inventory
Supplementary Table S9. ESAS Fatigue Severity by Insomnia, Depression, Anemia, and Medication Classes
Supplementary Table S10. Unadjusted and Adjusted Associations between Categories of ESAS Fatigue Severity at Baseline and Functional Recovery at 3 Months
Supplementary Figure S1. Recruitment of the study cohort
REFERENCES
- 1.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–856. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 2.Kahn JM, Benson NM, Appleby D, Carson SS, Iwashyna TJ. Long-term acute care hospital utilization after critical illness. JAMA. 2010;303:2253–2259. doi: 10.1001/jama.2010.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Needham DM, Bronskill SE, Calinawan JR, Sibbald WJ, Pronovost PJ, Laupacis A. Projected incidence of mechanical ventilation in Ontario to 2026: Preparing for the aging baby boomers. Crit Care Med. 2005;33:574–579. doi: 10.1097/01.ccm.0000155992.21174.31. [DOI] [PubMed] [Google Scholar]
- 4.A controlled trial to improve care for seriously ill hospitalized patients. The study to understand prognoses and preferences for outcomes and risks of treatments (SUPPORT). The SUPPORT Principal Investigators. JAMA. 1995;274:1591–1598. [PubMed] [Google Scholar]
- 5.Campbell ML, Guzman JA. Impact of a proactive approach to improve end-of-life care in a medical ICU. Chest. 2003;123:266–271. doi: 10.1378/chest.123.1.266. [DOI] [PubMed] [Google Scholar]
- 6.Curtis JR, Nielsen EL, Treece PD, et al. Effect of a quality-improvement intervention on end-of-life care in the intensive care unit: a randomized trial. Am J Respir Crit Care Med. 2011;183:348–355. doi: 10.1164/rccm.201006-1004OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Norton SA, Hogan LA, Holloway RG, Temkin-Greener H, Buckley MJ, Quill TE. Proactive palliative care in the medical intensive care unit: effects on length of stay for selected high-risk patients. Crit Care Med. 2007;35:1530–1535. doi: 10.1097/01.CCM.0000266533.06543.0C. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin MR, Wunsch H, Reyfman PA, et al. High burden of palliative needs among older intensive care unit survivors transferred to post-acute care facilities. a single-center study. Annals of the American Thoracic Society. 2013;10:458–465. doi: 10.1513/AnnalsATS.201303-039OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson JE, Curtis JR, Mulkerin C, et al. Choosing and Using Screening Criteria for Palliative Care Consultation in the ICU: A Report From the Improving Palliative Care in the ICU (IPAL-ICU) Advisory Board. Crit Care Med. 2013:9. doi: 10.1097/CCM.0b013e31828cf12c. [DOI] [PubMed] [Google Scholar]
- 10.Cox CE, Curtis JR. Using Technology to Create a More Humanistic Approach to Integrating Palliative Care into the Intensive Care Unit. American journal of respiratory and critical care medicine. 2016;193:242–250. doi: 10.1164/rccm.201508-1628CP. [DOI] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin MR, Reid MC, Westlake AA, et al. The feasibility of measuring frailty to predict disability and mortality in older medical intensive care unit survivors. Journal of critical care. 2014;29:401–408. doi: 10.1016/j.jcrc.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton Symptom Assessment System: a 15-year retrospective review of validation studies (1991–2006) Palliative medicine. 2008;22:111–122. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 14.Reddy S, Bruera E, Pace E, Zhang K, Reyes-Gibby CC. Clinically important improvement in the intensity of fatigue in patients with advanced cancer. Journal of palliative medicine. 2007;10:1068–1075. doi: 10.1089/jpm.2007.0007. [DOI] [PubMed] [Google Scholar]
- 15.Hui D, Shamieh O, Paiva CE, et al. Minimal Clinically Important Difference in the Physical, Emotional, and Total Symptom Distress Scores of the Edmonton Symptom Assessment System. Journal of pain and symptom management. 2016;51:262–269. doi: 10.1016/j.jpainsymman.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selby D, Chakraborty A, Myers J, Saskin R, Mazzotta P, Gill A. High scores on the Edmonton Symptom Assessment Scale identify patients with self-defined high symptom burden. J Palliat Med. 2011;14:1309–1316. doi: 10.1089/jpm.2011.0187. [DOI] [PubMed] [Google Scholar]
- 17.Casarett D, Karlawish J, Morales K, Crowley R, Mirsch T, Asch DA. Improving the use of hospice services in nursing homes: a randomized controlled trial. JAMA. 2005;294:211–217. doi: 10.1001/jama.294.2.211. [DOI] [PubMed] [Google Scholar]
- 18.Borson S, Scanlan J, Brush M, Vitaliano P, Dokmak A. The mini-cog: a cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. International journal of geriatric psychiatry. 2000;15:1021–1027. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Covinsky KE, Palmer RM, Counsell SR, Pine ZM, Walter LC, Chren MM. Functional status before hospitalization in acutely ill older adults: validity and clinical importance of retrospective reports. J Am Geriatr Soc. 2000;48:164–169. doi: 10.1111/j.1532-5415.2000.tb03907.x. [DOI] [PubMed] [Google Scholar]
- 20.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The indx of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 21.Ferrante LE, Pisani MA, Murphy TE, Gahbauer EA, Leo-Summers LS, Gill TM. Factors Associated with Functional Recovery Among Older ICU Survivors. American journal of respiratory and critical care medicine. 2016 doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hui D, Shamieh O, Paiva CE, et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: A prospective, multicenter study. Cancer. 2015;121:3027–3035. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herridge MS, Tansey CM, Matte A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 24.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369:1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davydow DS, Gifford JM, Desai SV, Needham DM, Bienvenu OJ. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434. doi: 10.1016/j.genhosppsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Archives of internal medicine. 2006;166:1993–1999. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 27.Unroe KT, Sachs GA, Dennis ME, et al. Hospice use among nursing home and non-nursing home patients. Journal of general internal medicine. 2015;30:193–198. doi: 10.1007/s11606-014-3080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168:1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 29.Huijberts S, Buurman BM, de Rooij SE. End-of-life care during and after an acute hospitalization in older patients with cancer, end-stage organ failure, or frailty: A sub-analysis of a prospective cohort study. Palliative medicine. 2016;30:75–82. doi: 10.1177/0269216315606010. [DOI] [PubMed] [Google Scholar]
- 30.Shega JW, Dale W, Andrew M, Paice J, Rockwood K, Weiner DK. Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc. 2012;60:113–117. doi: 10.1111/j.1532-5415.2011.03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahousse L, Ziere G, Verlinden VJ, et al. Risk of Frailty in Elderly With COPD: A Population-Based Study. The journals of gerontology Series A, Biological sciences and medical sciences. 2016;71:689–695. doi: 10.1093/gerona/glv154. [DOI] [PubMed] [Google Scholar]
- 32.Vaz Fragoso CA, Beavers DP, Hankinson JL, et al. Respiratory impairment and dyspnea and their associations with physical inactivity and mobility in sedentary community-dwelling older persons. J Am Geriatr Soc. 2014;62:622–628. doi: 10.1111/jgs.12738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaz Fragoso CA, Araujo K, Leo-Summers L, Van Ness PH. Lower Extremity Proximal Muscle Function and Dyspnea in Older Persons. Journal of the American Geriatrics Society. 2015;63:1628–1633. doi: 10.1111/jgs.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parentoni AN, Mendonca VA, Dos Santos KD, et al. Gait Speed as a Predictor of Respiratory Muscle Function, Strength, and Frailty Syndrome in Community-Dwelling Elderly People. J Frailty Aging. 2015;4:64–68. doi: 10.14283/jfa.2015.41. [DOI] [PubMed] [Google Scholar]
- 35.Serra-Prat M, Palomera E, Clave P, Puig-Domingo M. Effect of age and frailty on ghrelin and cholecystokinin responses to a meal test. The American journal of clinical nutrition. 2009;89:1410–1417. doi: 10.3945/ajcn.2008.27076. [DOI] [PubMed] [Google Scholar]
- 36.Ni Mhaolain AM, Fan CW, Romero-Ortuno R, et al. Frailty, depression, and anxiety in later life. International psychogeriatrics / IPA. 2012;24:1265–1274. doi: 10.1017/S1041610211002110. [DOI] [PubMed] [Google Scholar]
- 37.Brown PJ, Roose SP, Fieo R, et al. Frailty and depression in older adults: a high-risk clinical population. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2014;22:1083–1095. doi: 10.1016/j.jagp.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuen JK, Reid MC, Fetters MD. Hospital do-not-resuscitate orders: why they have failed and how to fix them. Journal of general internal medicine. 2011;26:791–797. doi: 10.1007/s11606-011-1632-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. Journal of pain and symptom management. 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. The oncologist. 2000;5:353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 41.de Rekeneire N, Leo-Summers L, Han L, Gill TM. Epidemiology of restricting fatigue in older adults: the precipitating events project. Journal of the American Geriatrics Society. 2014;62:476–481. doi: 10.1111/jgs.12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. The journals of gerontology Series A, Biological sciences and medical sciences. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Annals of internal medicine. 2001;135:313–321. doi: 10.7326/0003-4819-135-5-200109040-00007. [DOI] [PubMed] [Google Scholar]
- 44.Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respiratory medicine. 2012;106:522–530. doi: 10.1016/j.rmed.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 45.Baltzan MA, Scott AS, Wolkove N, et al. Fatigue in COPD: prevalence and effect on outcomes in pulmonary rehabilitation. Chronic respiratory disease. 2011;8:119–128. doi: 10.1177/1479972310396737. [DOI] [PubMed] [Google Scholar]
- 46.Radbruch L, Strasser F, Elsner F, et al. Fatigue in palliative care patients -- an EAPC approach. Palliative medicine. 2008;22:13–32. doi: 10.1177/0269216307085183. [DOI] [PubMed] [Google Scholar]
- 47.Gong S, Sheng P, Jin H, et al. Effect of methylphenidate in patients with cancer-related fatigue: a systematic review and meta-analysis. PLoS One. 2014;9:e84391. doi: 10.1371/journal.pone.0084391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hardy SE. Methylphenidate for the treatment of depressive symptoms, including fatigue and apathy, in medically ill older adults and terminally ill adults. The American journal of geriatric pharmacotherapy. 2009;7:34–59. doi: 10.1016/j.amjopharm.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.