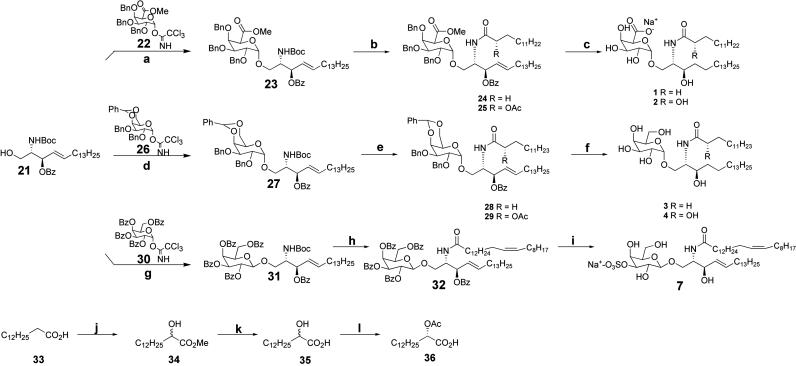
Fig. 4.
Synthesis of glycolipids. Reagents and conditions were as follows. a, 22, TMSOTf, 67%. b, (i) TFA, DCM; (ii) HBTU, myristic acid or (S)-2-acetoxymyristic acid, n-morpholine, ≈92%, two steps. c, H2, 20% Pd(OH)2, (ii) LiOH, H2O:THF:MeOH, 38%, two steps. d, 26, TMSOTf, 60%. e, (i) TFA, DCM, (ii) HBTU, myristic acid or (S)-2-acetoxymyristic acid, n-morpholine, ≈92%, two steps. f, (i) NaOMe, MeOH, (ii)Pd/C, H2, EtOH, 90%, two steps. g, 30, TMSOTf, 62%. h, (i) TFA, DCM, (ii) HBTU, nervonic acid, n-morpholine, 60%, two steps. i, (i) NaOMe, MeOH, quant; (ii) Bu2SnO, MeOH; (iii) Me3N·SO3, THF, 95%. j and k, (i) LDA, TMSOOTMS, (ii) H+, MeOH, 30%, two steps; then LiOH, H2O:MeOH, 81%; l, novozyme 435, CH2=CHOAc, 54% based on S isomer.