Figure 9.
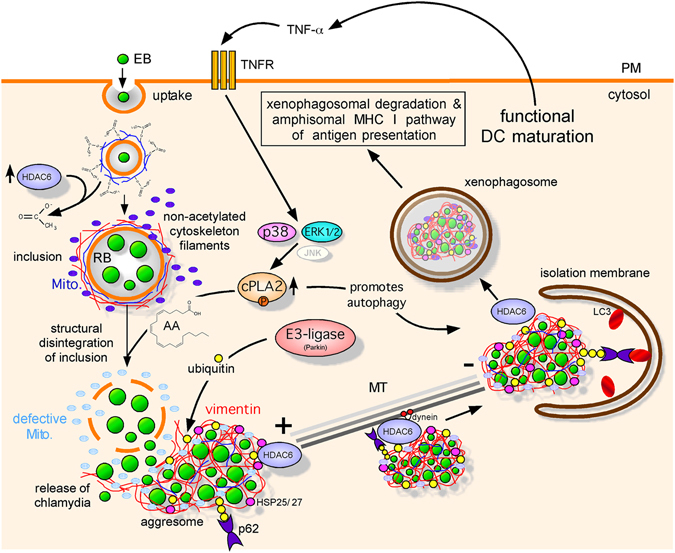
Model of mito-xenophagic degradation of bacterial structures in chlamydia-infected DCs. Infected DCs destroy inclusions resulting in the cytosolic release of bacteria. HDAC6-mediated deacetylation of stable MTs promotes inclusion disruption and a structural collapse of the associated vimentin. This is accompanied by a TNF-α mediated induction/activation of cPLA2, which catalyses the synthesis of AA. AA triggers disintegration of the fragile inclusions by affecting the proper function of inclusion-associated mitochondria. Since mitochondrial energy supply is essential for normal development and integrity of inclusions, a metabolic switch in infected DCs from OXPHOS to aerobic glycolysis causes irreversible damage to the bacteria. Chlamydial remnants are associated with collapsed vimentin, HDAC6, Parkin, small heat shock proteins (e.g. HSP25/27) as well as Pink-1-positive mito-aggresomes. Ubiquitinated aggresomal structures recruit p62, which binds to phagophore-conjugated LC3. Finally, xenophagosomes fuse with endo/lysosomal vacuoles. In parallel to the destruction of chlamydia, bacterial antigens are generated by the amphisomal pathway and are loaded onto MHC I derived from endosomal recycling. MHC I molecules successfully loaded with antigen are presented on the cell surface to CD8+ T cells.
