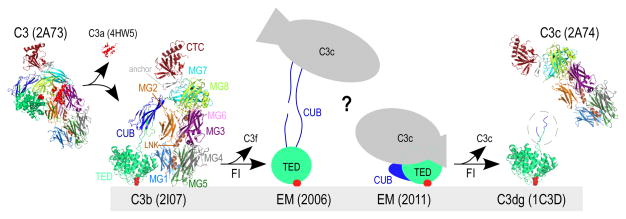
Figure 1. Structures of complement component C3 and activated products.
A simplified schematic of C3-mediated opsonization is shown. Transformation of inert C3 [PDB: 2A73 (2)] through cleavage by C3 convertases leads to the release of C3a [anaphylatoxin domain, PDB: 4HW5 (44)] and hydrolysis of the thioester bond (indicated with red circles). The product of this transformation is termed C3b [PDB: 2I07 (4)] and possesses marked conformational differences from its precursor molecule. C3b molecules close to cell surfaces attach via the activated thioester bond and tag them for opsonization. Sequential cleavages of C3b by FI yield iC3b, for which two controversial EM structures have been reported to date (depicted with cartoons drawn based on (16, 17)). Further cleavage of iC3b by FI yields two end products, surface-bound C3dg (the C3g sequence not crystallized in C3d is encircled, PDB: 1C3D (8)) and fluid-phase C3c (PDB: 2A74 (2)). Domain color annotation follows that in Janssen et al (2); PDB entries are shown in brackets.