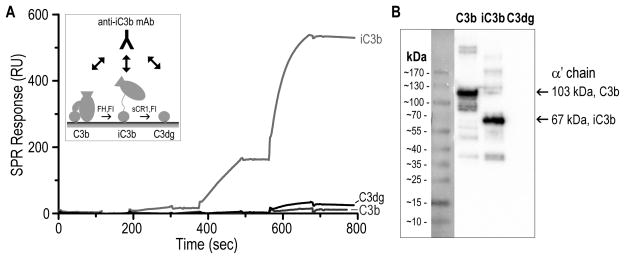
Figure 4. Binding specificity of anti-iC3b mAb for C3-derived opsonins.
A. For evaluating the specificity of the anti-iC3b mAb towards surface-bound opsonins, C3b was captured on a SPR sensor chip in physiological orientation via its thioester moiety. Individual C3b surfaces were converted to iC3b and C3dg with factor I (FI) and cofactors FH and sCR1, respectively (45) (insert). A titration experiment was performed on the C3b, iC3b and C3dg surfaces using consecutive injections of increasing anti-iC3b mAb concentrations (0.01–10 μg/ml). Anti-iC3b mAb showed a significant binding affinity for iC3b when compared to C3b and C3dg. The residual binding to C3dg that was observed was most likely due to iC3b residues present on the C3dg surface. B. Purified C3b, iC3b and C3dg were subjected to gel electrophoresis under reducing and denaturing conditions. Under reducing conditions, C3b produces two individual bands corresponding to the αβ- and β-chains, connected via a disulfide bond. Proteins were subsequently analyzed by western blotting using the anti-iC3b mAb. Intense bands detected for C3b and iC3b correspond to the α′-chains of the proteins (103 kDa for C3b and 67 kDa for iC3b, produced upon cleavage of C3f). C3dg did not show any detectable interaction with the anti-iC3b mAb.