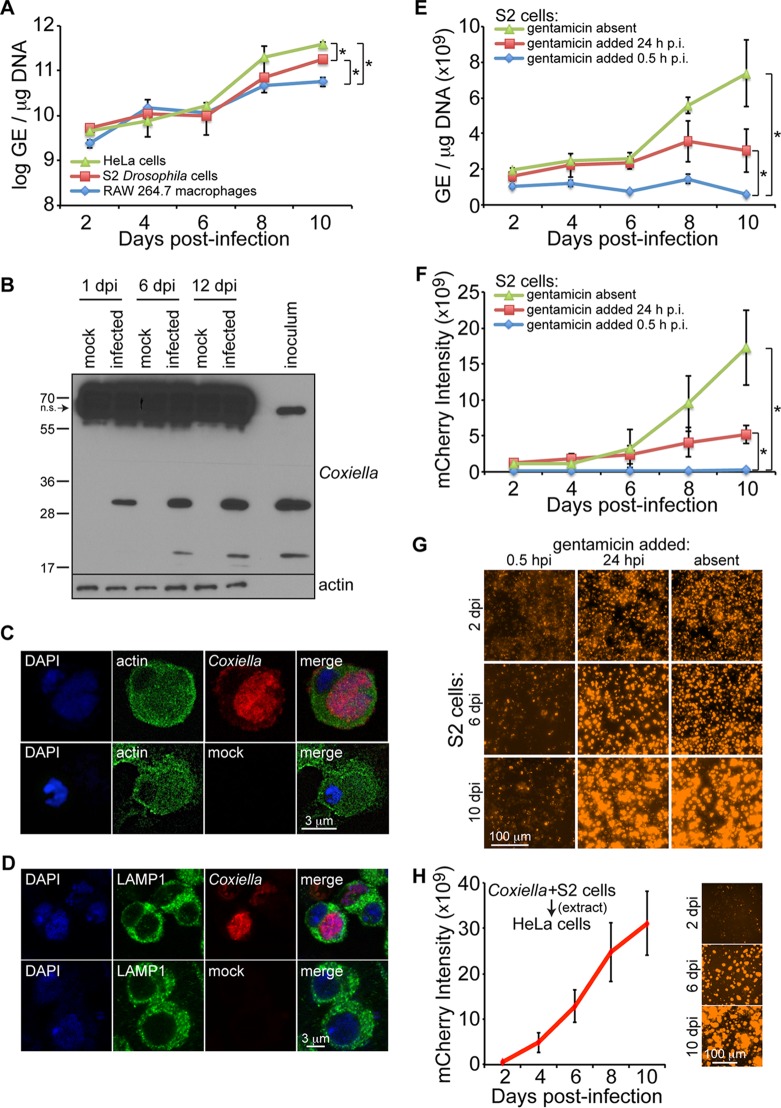
FIG 1.
C. burnetii replicates in Drosophila hemocyte-derived S2 cells. (A) Cells were infected (MOI = 100 GE/cell), and comparative growth kinetics of C. burnetii in insect and mammalian cells were determined by qPCR. The results are presented as log GE per microgram of DNA. (B) Immunoblotting detection of C. burnetii antigens in Drosophila S2 cells at 1, 6, and 12 days postinfection (dpi) using a rabbit polyclonal antibody against Coxiella. Nonspecific (n.s.) banding in S2 cell lysates is denoted by the arrow. (C and D) Drosophila hemocyte-derived S2 cells were infected with C. burnetii expressing mCherry and prepared for confocal microscopy at 4 dpi. Nuclei were stained with DAPI and actin (C) or LAMP1 (D). (E to G) A gentamicin protection assay was performed to evaluate the growth of mCherry-expressing C. burnetii in Drosophila S2 cells. (E and F) At the indicated times postinfection, total DNA was collected to determine GE levels (E) or mCherry intensity was measured at five different locations of three independent wells at the indicated time points postinfection (F). (G) Representative images for each condition at 2, 6, and 10 days postinfection. (H) mCherry-expressing Coxiella was isolated from infected S2 cells and used to infect HeLa cells at an MOI of 100 GE/cell. The intensity of mCherry was measured over the course of 10 days, and representative images are shown. The asterisks denote statistical significance (*, P < 0.05). The error bars indicate standard deviations.