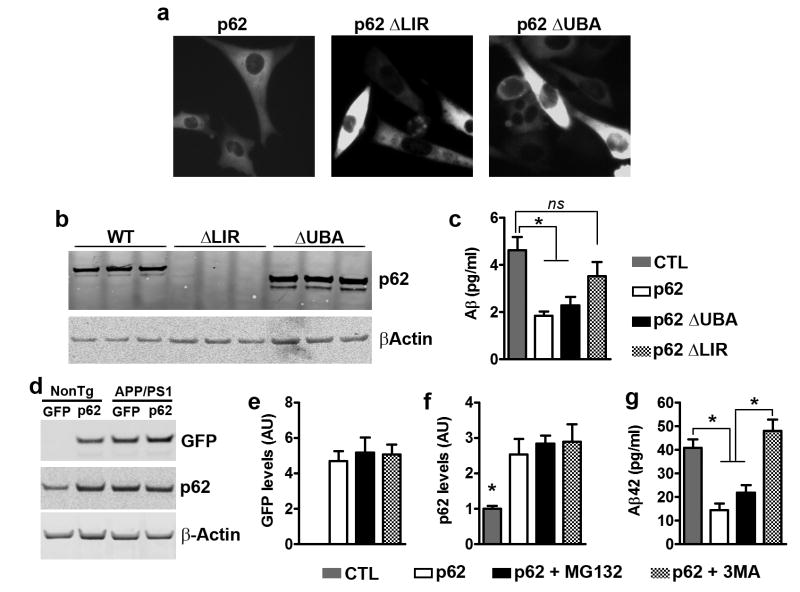
Figure 5.
p62 overexpression decreases Aβ by an autophagy-mediated mechanism. (a) Microphotographs of 7PA2 cells transfected with different p62 constructs, as indicated. (b) Western blot from proteins isolated from transfected 7PA2 cells and probed with a p62 antibody, which recognize p62 within the LIR domain. Therefore ΔLIR is not detectable, while there is a clear shift in molecular weight for ΔUBA. (c) Aβ levels obtained from 7PA2 cells and measured by sandwich ELISA were different among the four groups (p = 0.001). Post hoc analyses revealed that transfection of wild type p62 or p62-ΔUBA decreased Aβ42 levels. In contrast, p62-ΔLIR was unable to significantly reduce Aβ levels. (d) Western blot of proteins extracted from APP/PS1 primary neurons infected with the p62 AAVs and treated with compounds to block autophagy (3MA) or proteasome function (MG132), as indicated. (e) GFP levels were similar among the three groups of neurons infected with the p62 AAVs. (f) p62 blot showed that p62 levels were significantly higher in the three groups infected with the p62 AAVs, compared to uninfected neurons. (g) Aβ42 levels obtained from these APP/PS1 neurons and measured by sandwich ELISA, were significantly different among the four groups (p < 0.0001). Post hoc analyses revealed that the p62 and the p62 + MG132 groups had significantly lower Aβ42 levels compared to the other two groups. For all the experiments shown here, n=9 mice per group. Quantitative analyses of the blots were performed by normalizing the levels of the protein of interest to β-actin, used as a loading control. Data were analyzed by one-way ANOVA and Bonferroni’s post hoc test and are presented as mean ± SEM.