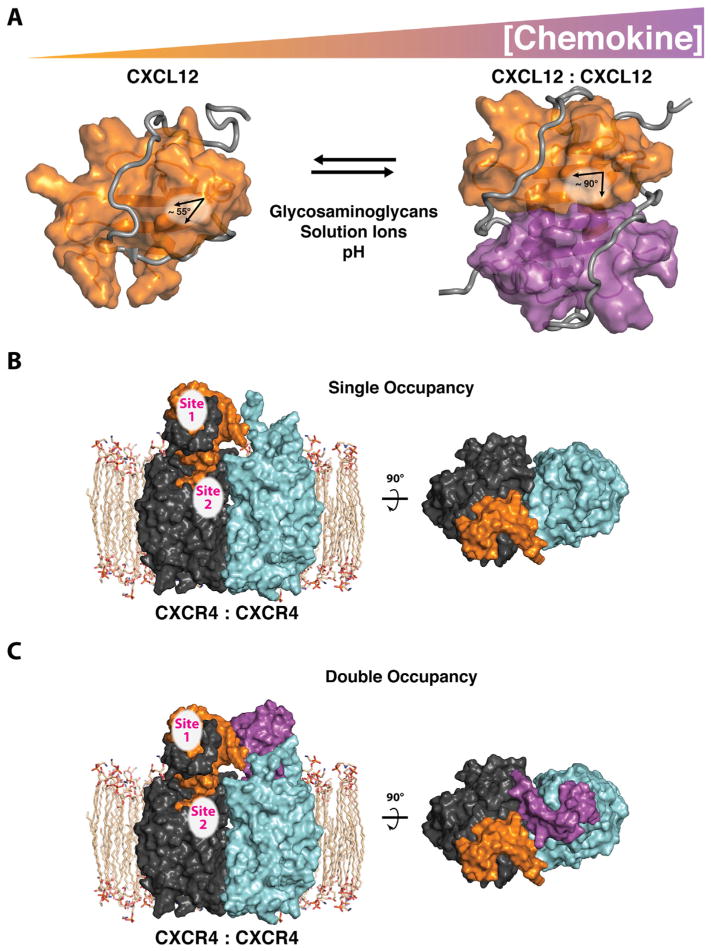
Figure 2.
Chemokine oligomerization influences receptor occupancy. (A) Chemokine concentration and environmental conditions such as glycosaminoglycans, solution ions, pH, and soluble receptor peptides (gray), regulate chemokine self-association. The C-terminal α-helix of CXCL12 is rearranged (helix angle: 55–90°) upon CXC-dimer formation at the first β-strand of each monomeric protein (shown in orange and purple). Important receptor binding epitopes (site 1) remain available on each CXCL12 monomeric subunit, irrespective of oligomeric state (PDB IDs: 2N55 and 2K05). (B) Monomeric CXCL12 forms a single-occupancy (2:1) complex with the CXCR4 receptor dimer (receptor monomers are shown as dark and light gray). (C) Monomeric CXCL12 could form a double occupancy (2:2) complex (shown). A double occupancy (2:2) complex, between dimeric CXCL12 and the CXCR4 dimer, while maintaining important sites 1 and 2 contacts, is a possible alternative mode of receptor binding (not shown).