Abstract
Background
Local control remains a challenge in pediatric parameningeal rhabdomyosarcoma (PM-RMS), and survival after local failure (LF) is poor. Identifying patients with a high risk of LF is of great interest to clinicians. In this study, we examined whether tumor response to induction chemotherapy (CT) could predict LF in embryonal PM-RMS.
Methods
We identified 24 patients with embryonal PM-RMS, age 2 to 18 years, with complete magnetic resonance imaging and gross residual disease after surgical resection. All patients received proton radiation therapy (RT), median dose 50.4 GyRBE (50.4-55.8 GyRBE). Tumor size was measured before initial CT and before RT.
Results
With a median follow-up time of 4.1 years for survivors, LF was seen in 9 patients (37.5%). The median time from the initiation of CT to the start of RT was 4.8 weeks. Patients with LF had a similar initial (pre-CT) tumor volume compared with patients with local controlled (LC) (54 cm3 vs 43 cm3, P=.9) but a greater median volume before RT (pre-RT) (40 cm3 vs 7 cm3, P=.009) and a smaller median relative percent volume reduction (RPVR) in tumor size (0.4% vs 78%, P<.001). Older age (P=.05), larger pre-RT tumor volume (P=.03), and smaller RPVR (P=.003) were significantly associated with actuarial LF on univariate Cox analysis.
Conclusions
Poor response to induction CT appears to be associated with an increased risk of LF in pediatric embryonal PM-RMS.
Introduction
Parameningeal rhabdomyosarcoma (PM-RMS) represents a distinct challenge in pediatric oncology. Disease control is poor compared with other RMS sites, with a 10-year overall survival (OS) of 66% for all PM patients and survival rates as low as 52% for patients with multiple adverse risk factors (1). Local progression remains the greatest impediment to cure in these patients. Local failure (LF) in PM-RMS is seen at a rate of 19% and accounts for 68% of all PM failures (1, 2). Moreover, survival rates after recurrence are extremely poor, ranging from 0% to 20%, making effective initial treatment critical (3-5). Therefore, great interest lies in the identification of PM patients who may benefit from treatment intensification.
Much effort has been put toward identifying harbingers of LF in PM-RMS. Pretreatment characteristics such as age, histology, intracranial extension (ICE), large tumor size, and unfavorable PM location have been variably predictive of poorer outcomes (1, 2, 6, 7); however, not all patients with these features will experience failure, and designation of these patients as being at high risk could lead to overtreatment in many cases. Response to induction chemotherapy (CT) is an ideal choice for assessing local and disease failure risk but has generated conflicting conclusions in the literature. Publications from Ferrari et al (6) and Dantonello et al (8), looking at large Italian and German cohorts respectively, found that tumor response to initial CT was predictive of OS and event-free survival (EFS) in pediatric RMS. Dharmarajan et al (9) found a nearly significant improvement in LF in RMS patients with negative positron emission tomography (PET) imaging after induction CT (3% vs 19%, P=.06), suggesting that response to CT may influence local control (LC) as well. By contrast, separate analyses of 2 Children's Oncology Group (COG) trials have failed to show a correlation between response to induction CT and failure-free survival (FFS) (10, 11).
In this study of patients with embryonal PM-RMS, we evaluated radiographic response to induction CT to determine whether response correlated with LC, which is critical to the survival of these children and a departure from the prior European and COG studies that have used FFS and OS as endpoints. We also investigated the relationship with FFS. We limited our study population to PM-RMS with embryonal histology because our clinical observation has been that the patients with embryonal PM-RMS with a poor response to CT are at higher risk of experiencing LF, whereas patients with alveolar disease typically respond well, but more commonly experience distant failure.
Patients and Methods
Patients
We retrospectively identified 25 consecutive patients with intermediate risk (as defined by COG D9803 and COG ARST 0531) embryonal PM-RMS, ages 2 to 18 years, treated at Massachusetts General Hospital between 2004 and 2012. Twenty-four patients had magnetic resonance imaging (MRI) scans available for 3-dimensional measurements at the desired time points and were included in the study. The excluded patient did not have a pre-CT MRI.
Treatment
All included patients had significant measurable disease at the start of induction CT. Nearly all patients treated before 2008 received CT according to the COG D9803 regimen, whereas from 2008 forward, the majority were treated according to COG ARST 0531. Four international patients received CT on the European EpSSG 2005 or MMT-98 protocol.
All patients received passively scattered proton radiation (RT) at the Francis H. Burr Proton Center, Massachusetts General Hospital with doses ranging from 50.4 to 55.8 Gy. The proton dose was prescribed in GyRBE units, using a relative biologic effectiveness (RBE) of 1.1 (12). Treatment volumes conformed to COG protocol specifications. A cone down after 36 Gy was used in accordance with the COG RMS protocol guidelines (11). The timing of RT was determined either by protocol or in consultation with the referring doctors.
Tumor measurement and response evaluation
Tumor volumes were assessed by MRI in all patients. Tumors were measured by a single radiation oncologist, blinded to patient outcomes, before induction CT (pre-CT) and before RT (pre-RT). The dimensions of each tumor were measured in 3 planes (width, depth, height) using the maximum perpendicular diameters. Ellipsoid tumor volumes were calculated using the formula 4/3π (r1 × r2 × r3). The absolute volume reduction (AVR) was calculated by subtracting the pre-RT volume from the pre-CT volume, and the relative percent volume reduction (RPVR) was derived using the following equation:
Prior COG analyses of RMS response to CT used the World Health Organization (WHO) criteria for tumor measurement, which uses the product of the maximum perpendicular diameters in the axial plane only (13). To facilitate comparison, response was also calculated using the 2-dimensional WHO method. A complete response (CR) was defined as complete resolution of disease. A partial response (PR) was defined as a decrease of 50% or more in the product of the maximum perpendicular diameters of the tumor. No response (NR) was considered less than a 50% decrease and less than a 25% increase in the product of the maximum perpendicular diameters, and progressive disease (PD) was defined as a 25% or greater increase in the product of the maximum perpendicular diameters. Examples of radiographic response to CT are shown in Figure 1. Volumetric measurements produce a greater percent change in tumor size compared with the 2-dimensional WHO method, and conversions are provided in the WHO report (13). For reader orientation, a 50% decrease and a 25% increase in estimated tumor volume by the 2-dimensional WHO method are equal to a 65% decrease and a 40% increase by volumetric estimates.
Fig. 1.
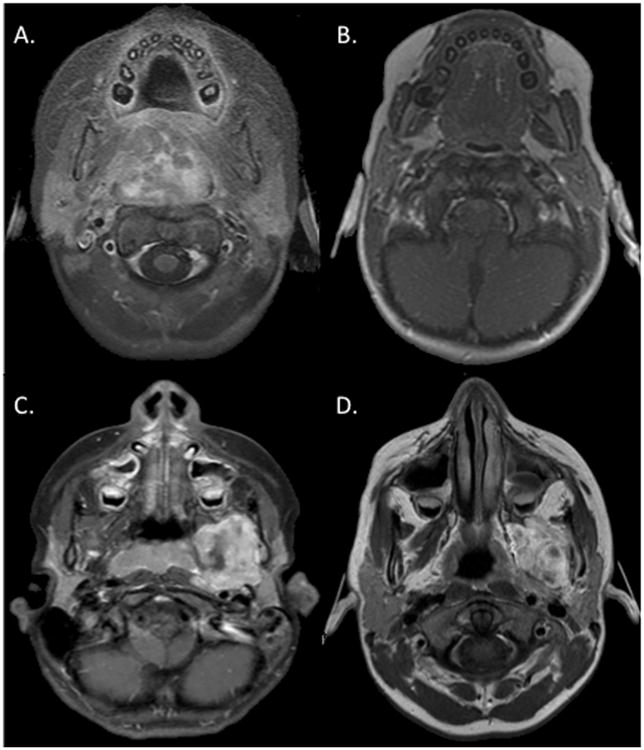
Varying response to chemotherapy for embryonal parameningeal rhabdomyosarcoma. (A) Prechemotherapy magnetic resonance imaging (MRI) for a nasopharyngeal patient with a 39-cm3 tumor. (B) MRI after 4 weeks of chemotherapy with 0.9 cm3 residual and 98% reduction in tumor volume. (C) Prechemotherapy MRI for a parapharyngeal primary and 27-cm3 tumor. (D) MRI after 13 weeks of chemotherapy with tumor volume decreased by 2%. The nasopharyngeal patient continues to experience local control. The parapharyngeal patient experienced local failure 6 months after radiation.
Statistical methods
The primary endpoints of this study were LC and tumor response. Disease endpoints of FFS, OS, and LC were measured from the date of RT start. The failure event for FFS was the earliest date of local, regional, or metastatic failure, or death. Patients who had not experienced failure with the relevant event were censored at the date of their last follow-up visit. Actuarial rates of FFS, OS, and LC were estimated by the Kaplan-Meier method. Log-rank test, Fisher exact test, and Wilcoxon rank-sum test were used to compare LF and LC groups and to compare responders with nonresponders. The P values were based on 2-sided tests. All calculations were performed using Stata (Stata-Corp. 2013; Stata Statistical Software: Release 13; College Station, TX).
Results
The patient characteristics are reported in Table 1. The median age at initiation of CT was 5.2 years (range, 2.0-15.3 years). ICE was seen in 13 (59%) patients and regional nodal disease in 5 patients (21%). The median time from CT initiation to RT start was 5 weeks (range, 3-20 weeks), and the median interval between pre-CT MRI and pre-RT MRI was 6 weeks (range, 4-20 weeks). The median duration of RT was 41 days (range, 36-52 days).
Table 1. Patient characteristics (N = 24).
| Characteristic | n |
|---|---|
| Age | |
| Median (range), y | 5.2 (2.0-15.3) |
| Sex | |
| Male | 13 (54%) |
| Group | |
| III | 22 (92%) |
| IV | 2 (8%) |
| Stage | |
| II | 7 (29%) |
| III | 15 (63%) |
| IV | 2 (8%) |
| Histology | |
| Embryonal | 24 (100%) |
| Risk group | |
| Intermediate | 22 (92%) |
| High | 2 (8%) |
| Parameningeal site | |
| Parapharyngeal | 5 (21%) |
| Nasopharyx | 5 (21%) |
| Masticator space | 5 (21%) |
| Paranasal/sinus | 4 (17%) |
| Infratemporal fossa | 3 (12%) |
| Auditory canal | 2 (8%) |
| Nodal disease | |
| N0 | 19 (79%) |
| N1 | 5 (21%) |
| Size | |
| Median (range), cm3 | 43 (3-272) |
| ≤5 cm | 11 (46%) |
| >5 cm | 13 (54%) |
| Intracranial extension | |
| Absent | 10 (42%) |
| Present | 14 (58%) |
| Surgery | |
| Biopsy only | 22 (92%) |
| Subtotal resection | 2 (8%) |
| Chemotherapy regimen* | |
| ARST 0531 | 12 (51%) |
| COG D9803 | 7 (29%) |
| EpSSG 2005 | 3 (12%) |
| Other | 2 (8%) |
| Radiation dose | |
| Median (range) | 50.4 Gy (50.4-55.8 Gy) |
ARST0531, VAC v. VAC VI; COG D9803; VAC v. VTC; EpSSG 2005, VAIf +/- D, where V=vincristine, A=actinomycin, C=cyclo-phosphamide, I=Irinotecan, If=Ifosfamide; D=Doxorubicin.
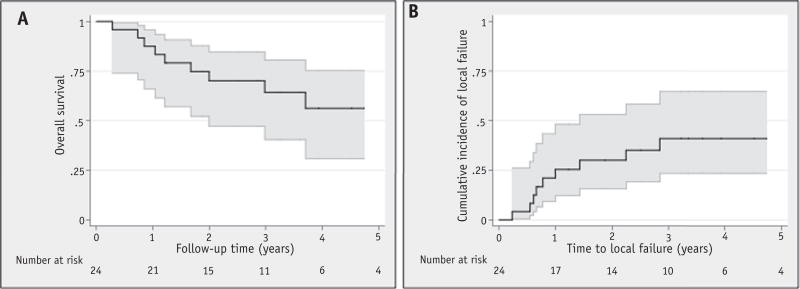
The median follow-up time was 3.1 years (range, 0.3-8.6 years) for all patients and 4.1 years (range, 1.2-8.6 years) for survivors. The actuarial 3-year FFS, OS, and LC were 52% (95% confidence interval [CI], 30%-70%), 64% (95% CI, 40%-80%), and 59% (95% CI, 24%-65%), respectively (Fig. 2). The median time to failure was 11 months from RT start (range, 3-47 months). There were 12 patients who experienced failure: 9 with a local component (7 with LF only, 1 with LF and distant failure,and 1 with LF and regional failure), 2 with regional failures, and 1 with distant failure. All local recurrences within the high RT dose region without marginal failures. No differences in gross tumor volume or clinical target volume (CTV) coverage were observed between LC and LF patients (median CTV95 was 100% for both groups). All patients had CTV coverage by the prescription dose of 98% or higher. The 2 isolated regional failures occurred in cervical nodes well outside the radiation portals.
Fig. 2.
Overall survival (A) and local failure (B) for the entire cohort.
For all patients, the median pre-CT tumor volume was 43 cm3 (range, 3-272 cm3), and the median time from pre-CT MRI to CT start was 7 days (range, 16 days before CT to 2 days after CT start). The median pre-RT tumor volume was 26 cm3 (range, 0-141 cm3), and the median time from pre-RT MRI to RT start was 11 days (range, 1-14 days before RT). The results for all patients are shown in Table 2.
Table 2. Tumor characteristics, response, and outcomes for all patients.
| Group | Sex | Age, y | Subsite | Nodes | ICE | Surgery | Chemotherapy | RT dose (CGE) |
CT to RT start (wks) |
Pre-CT Vol. (cm3) |
Pre-RT Vol. (cm3) |
AVR (cm3) | RPVR (%) | WHO response (%) |
Failure status | Time to failure (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Local failure | M | 15.3 | Masticator | N0 | - | Biopsy | ARST 0531 | 50.4 | 5 | 31 | 36 | −5 | −16 | − 17 (NR) | LF | 12 |
| M | 2.0 | Nasopharynx | N0 | - | Biopsy | COG D9803 | 50.4 | 12 | 28 | 32 | −4 | −14 | 31 (NR) | LF | 8 | |
| M | 5.1 | Masticator | N1 | - | Biopsy | ARST 0531 | 50.4 | 13 | 62 | 66 | −4 | −6 | − 14 (NR) | LF | 7 | |
| F | 7.9 | Parapharyngeal | N0 | - | Biopsy | COG D9803 | 50.4 | 4 | 6 | 6 | 0 | 0 | 0 (NR) | LF | 27 | |
| F | 7.5 | Parapharyngeal | N0 | + | Biopsy | ARST 0531 | 54 | 4 | 85 | 85 | 0 | 0 | 0 (NR) | LF | 9 | |
| M | 7.4 | Parapharyngeal | N1 | + | Biopsy | EpSGG | 55.8 | 13 | 27 | 27 | 0 | 2 | 2 (NR) | LF & RF | 7 | |
| M | 10.5 | Nasopharynx | N0 | + | Biopsy | ARST 0531 | 50.4 | 4 | 67 | 59 | 8 | 12 | 4 (NR) | LF | 17 | |
| F | 7.5 | Masticator | N1 | - | Biopsy | COG D9803 | 50.4 | 11 | 54 | 40 | 14 | 27 | 17 (NR) | LF & DM | 3 | |
| M | 4.1 | Infratemporal | N0 | + | Biopsy | MSK 03099 | 50.4 | 19 | 62 | 41 | 21 | 33 | 25 (NR) | LF | 34 | |
|
| ||||||||||||||||
| Median | 7.5 | - | - | - | - | - | 50.4 | 11 | 54 | 40 | 0.3 | 0.4 | 2 | - | 9 | |
|
| ||||||||||||||||
| Local control | M | 8.8 | Infratemporal | N0 | + | Biopsy | ARST 0531 | 50.4 | 4 | 53 | 81 | −29 | −54 | −16 (NR) | NED | N/A |
| F | 4.1 | Paranasal | N1 | + | STR | ARST 0531 | 52.2 | 5 | 58 | 32 | 26 | 45 | 42 (NR) | NED | N/A | |
| M | 2.3 | Infratemporal | N0 | + | Biopsy | COG D9803 | 50.4 | 3 | 43 | 23 | 20 | 47 | 49 (NR) | NED | N/A | |
| M | 7.4 | Masticator | N0 | + | Biopsy | ARST 0531 | 50.4 | 5 | 43 | 20 | 23 | 53 | 37 (NR) | DM | 3 | |
| F | 3.4 | Nasopharynx | N0 | + | Biopsy | ARST 0531 | 50.4 | 4 | 99 | 42 | 57 | 58 | 44 (NR) | RF | 47 | |
| F | 3.6 | Masticator | N0 | + | Biopsy | ARST 0531 | 50.4 | 5 | 72 | 25 | 46 | 65 | 52 (PR) | NED | N/A | |
| F | 2.5 | Middle ear | N0 | + | Biopsy | COG D9803 | 50.4 | 4 | 27 | 7 | 20 | 74 | 53 (PR) | NED | N/A | |
| M | 2.8 | EAC | N0 | + | Biopsy | ARST 0531 | 50.4 | 4 | 23 | 5 | 18 | 78 | 60 (PR) | NED | N/A | |
| F | 2.5 | Parapharyngeal | N1 | + | Biopsy | MMT- 98 | 50.4 | 20 | 160 | 32 | 128 | 80 | 62 (PR) | NED | N/A | |
| F | 5.3 | Maxillary Sinus | N0 | - | Biopsy | ARST 0531 | 50.4 | 9 | 30 | 5 | 25 | 83 | 57 (PR) | NED | N/A | |
| F | 7.5 | Paranasal | N0 | - | Biopsy | ARST 0531 | 50.4 | 5 | 2 | 0 | 2 | 84 | 57 (PR) | NED | N/A | |
| F | 6.1 | Parapharyngeal | N0 | + | Biopsy | EpSGG | 50.4 | 20 | 17 | 2 | 15 | 87 | 70 (PR) | NED | N/A | |
| M | 2.5 | Nasopharynx | N0 | - | Biopsy | EpSGG | 50.4 | 17 | 9 | 1 | 8 | 87 | 84 (PR) | RF | 15 | |
| M | 2.4 | Nasopharynx | N0 | - | Biopsy | ARST 0531 | 50.4 | 4 | 39 | 1 | 38 | 98 | 92 (PR) | NED | N/A | |
| M | 10.0 | Ethmoid sinus | N0 | - | STR | COG D9803 | 50.4 | 15 | 272 | 0 | 272 | 100 | 100 (CR) | NED | N/A | |
|
| ||||||||||||||||
| Median | 3.6 | - | - | - | - | - | 50.4 | 5 | 43 | 7 | 23 | 78 | 57 | - | 15 | |
|
| ||||||||||||||||
| P value | .4 | .09 | - | .2 | .3 | - | - | .06 | .8 | .9 | .008 | .004 | .0005 | .0004 | - | - |
Abbreviations: AVR = absolute volume reduction; CE = intracranial extension; CR = complete response; CT = chemotherapy; DM = distant metastases; EAC = external auditory canal; LF = local failure; NR = no response; PR = partial response; RF = regional failure; RPVR = relative percent volume reduction; RT = radiation therapy; STR = subtotal resection.
CT to RT Start represents the time from chemotherapy initiation to radiation therapy start in weeks.
P values correspond to differences between local failure and local control groups.
The initial tumor size (pre-CT volume) for patients with LF and LC was similar, with a median of 54 cm3 for the LF group and 43 cm3 for the LC group (P=.9). By contrast, tumor response to induction CT was strongly associated with LC. The median postinduction CT (pre-RT) tumor volume was 40 cm3 for the LF group versus 7 cm3 for the LC group (P=.008). Similarly, the median AVR was 0.3 cm3 for the LF group versus 22 cm3 for the LC group (P=.004), and the median RPVR was 0.4% for the LF group and 78% for the LC group (P=.0005). Tumor response did not vary based on the duration of CT before RT. The median RPVR for patients who underwent RT at week 5 or earlier was 50% versus 57% for patients for patients who underwent RT after week 5 (P=.5). When patient response was examined according to the WHO criteria (the change in the product of the maximum perpendicular tumor diameters), the objective response rate was 42% with 1 CR, 9 PR, and 14 patients having NR (Table 2).
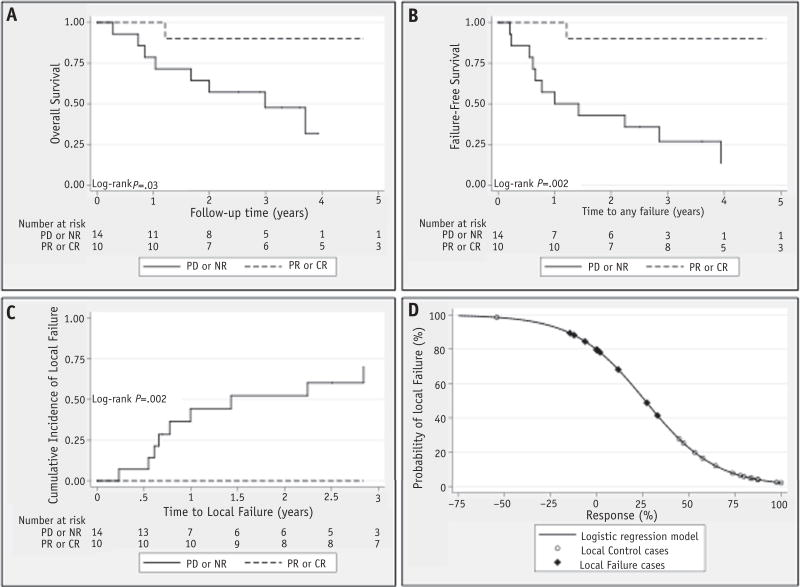
Survival curves for OS, FFS, and LF based on response are shown in Figure 3. FFS, LF, and OS were significantly better in those patients who achieved a CR or a PR. A logistic regression model (Fig. 3) was generated to assess the probability of LF as a function of the RPVR after CT. For patients with a 0% reduction in tumor volume after CT, there was an 80% chance of LF. The risk of failure dropped to 40% with a 33% reduction in tumor volume, 10% with a 66% reduction in volume, and 2% with a CR.
Fig. 3.
(A) Overall survival, (B) failure-free survival, and (C) local failure by World Health Organization tumor response. (D) Logistic regression model for the probability of local failure based on the relative percent volume reduction after induction chemotherapy. CR = complete response; NR = no response; PD = progressive disease; PR = partial response.
Using univariate Cox regression, factors associated with local LF and FFS were examined (Table 3). Increasing age (P=.05), a greater pre-RT tumor volume (P=.03), a smaller AVR (P=.04), a smaller RPVR (P=.003), and a tumor response of NR or PD (P=.01) were significantly associated with LF. For FFS, only RPVR (P = .01) and a response of NR or PD (P= .05) were significant predictors, although larger pre-RT tumor volume was of borderline significance (P = .06). Factors including sex, initial (pre-CT) tumor volume, CT to pre-RT MRI interval, CT to RT interval, nodal status, and intracranial extension did not influence LF or FFS.
Table 3. Univariate Cox regression analysis of local failure and failure-free survival.
| Local failure | Failure-free survival | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Characteristic | Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value |
| Age (y) | 1.18 | 1.00-1.39 | .05 | 1.13 | 0.97-1.32 | .11 |
| Sex | 0.57 | 0.14-2.27 | .42 | 0.50 | 0.15-1.68 | .26 |
| ICE | 0.48 | 0.13-1.80 | .28 | 0.57 | 0.18-1.77 | .33 |
| Nodes | 3.35 | 0.83-13.6 | .09 | 2.02 | 0.54-7.56 | .30 |
| Pre-CT volume, cm3 | 0.99 | 0.98-1.01 | .48 | 0.99 | 0.98-1.01 | .35 |
| Pre-RT volume, cm3 | 1.03 | 1.00-1.05 | .03 | 1.02 | 1.00-1.04 | .06 |
| CT to pre-RT MRI interval, wk | 1.02 | 0.92-1.12 | .75 | 1.02 | 0.94-1.10 | .68 |
| CT to RT interval, wk | 1.02 | 0.92-1.12 | .71 | 1.02 | 0.93-1.11 | .67 |
| AVR, cm3 | 0.97 | 0.94-0.99 | .04 | 0.98 | 0.95-1.00 | .09 |
| RPVR, % | 0.98 | 0.97-0.99 | .003 | 0.98 | 0.97-0.99 | .01 |
| Response type (PR or CR vs PD or NR) | - | - | - | 0.08 | 0.009-0.60 | .02 |
Abbreviations: CI = confidence interval; CR = complete response; ICE = intracranial extension; AVR = absolute volume reduction; NR = no response; PD = progressive disease; PR = partial response; RPVR = relative percent volume reduction.
CT to pre-RT MRI represents the time from chemotherapy initiation to preradiation therapy MRI in weeks.
CT to RT interval represents the time from chemotherapy initiation to radiation start in weeks.
The hazard ratio for local failure based on response type could not be calculated because of the absence of any local failures in patients with CR or PR.
Discussion
These data demonstrate a strong link between tumor response to induction CT and LC in intermediate-risk embryonal PM-RMS. Almost uniformly, patients with limited tumor regression (RPVR of ≤33%) experienced LF, and those with regression of ≥45% remained with locally controlled disease. Pretreatment factors including initial tumor volume, sex, nodal disease, and ICE did not correlate with LF, whereas pre-RT tumor volume was a significant predictor, likely acting as a surrogate for tumor response. Interestingly, a longer duration of induction CT did not increase tumor response. Our results suggest that tumor response occurs quickly during induction therapy and that patients could be reliably assessed as early as week 5, rather than at week 8, 9, or 12 as has been done in prior studies, which may have implications for future RMS trials (6, 10, 11).
The Italian cohort from Ferrari et al (6) showed a similar connection between tumor reduction and OS. More recently, Dantonello et al (8) found that EFS was significantly worse for German children with group III embryonal RMS who had stable disease or PD after induction CT (5-year EFS of 68% for responders vs 36% for SD/PD, P<.001). This correlation between response and survival was seen in earlier German cooperative group soft tissue sarcoma studies as well (14-16). However, the 2 prior COG analyses did not find such a correlation (10, 11). Although they were large in scope and inclusive of all sites, neither COG analysis examined embryonal PM-RMS patients separately nor looked at LC as an endpoint. Given the variability of prognosis based on site within pediatric RMS, it is plausible that response to CT may be of significance only in certain primary locations, such as the parameninges. To this point, in the German study by Dantonello et al (8), tumor response to induction CT of <33% predicted for significantly worse EFS in the patients with PM (65% vs 50%, P=.07) and non-PM head and neck (71% vs 0%, P<.001) embryonal disease, whereas the significance was not retained in any other subsite. Furthermore, LC was not examined in any of these studies, and the use of FFS as an endpoint could potentially diminish the correlation between tumor regression and LC through the inclusion of regional and distant failures. Indeed, in our study, 3 patients with excellent tumor regression (53%, 58%, and 87%) experienced sustained LC but experienced regional or distant failure. Still, FFS was strongly correlated with tumor response after induction CT in our study. It would be interesting to see whether FFS retained significance for embryonal PM-RMS in subsite analysis of the COG cohorts.
The use of a continuous model of response, implemented in our study and the study by Ferrari et al (6), may be a more sensitive method of assessing survival outcomes than the categorical methods used in the COG analyses, especially for patients with intermediate volume reductions (≤50%) after CT. Our logistic regression model (Fig. 3) predicts the chance of LC to be 88% with a 65% tumor reduction by volume, 20% for those with no tumor reduction, and 3% for those with a 40% increase in volume. The WHO designation of a 50% reduction to a 25% increase as “No Response” (volumetrically equivalent to a 65% decrease to a 40% increase) could incorporate patients with widely differing LF risks into a single prognostic group and may not be useful in predicting potential outcomes for PM-RMS patients.
Initial tumor volume did not predict for LF or FFS in our cohort, although it was predictive of OS in the studies by Ferrari et al (6) and Dantonello et al (8). Compared with the Italian study, pretreatment volumes for embryonal RMS were similar: 37 cm3 (range, 28-50 cm3) in the series by Ferrari et al (6) and 42 cm3 (range, 3-272 cm3) in our cohort. Heterogeneity between the 2 results could be attributed to differences in risk based on RMS site and histology, inasmuch as neither PM sites nor embryonal histology were examined individually in the Italian cohort, or to the small sample size in our study. In an analysis of predictors of LF in group III RMS patients from IRS-III, Wharam et al (17) found that initial tumor size ≥5 cm was predictive of FFS but not of LC. Dharmarajan et al (9) found that LC was not associated with initial tumor size but correlated with a complete response by PET (P=.06). Furthermore, the COG analysis by Burke et al (10) did not show a difference in response rates to induction CT by tumor size, supporting our findings that tumor response, not tumor size, is the driving factor for LF in PM-RMS.
In our study, LF was 41% (95% CI, 23%-65%), nearly double that in PM-RMS patients from the COG IRS-IV and D9803 trials (19%) (2). Target coverage was evaluated and exceeded COG minimum standards, and there were no marginal failures. Importantly, though, the objective response rate (tumor reduction of ≥50%) in these COG studies was 81% to 85%, and in our cohort it was approximately half that at 42%. It is likely that the higher rate of LF seen in our cohort is attributable to the poorer response to CT. Other possible explanations include a bias toward referring large, poorly responding tumors for proton therapy, given that the geometry of these tumors in young children can be challenging with regard to sizable photon treatment planning volumes and late toxicities. The use of proton therapy itself in these patients is unlikely to have led to this higher rate of LF. We have recently published the 5-year outcomes from our prospective multi-institutional proton study of pediatric RMS and have found that the outcomes were comparable with those in the most recent COG studies (18). Furthermore, the use of protons in other pediatric tumor types such as medulloblastoma, low-grade glioma, Ewing sarcoma, ependymoma, chordoma, and others has shown equivalent if not favorable disease outcomes (19-23).
Radiation therapy plays a central role in LC in PM-RMS, and radiation dose escalation may be an option for treatment intensification in patients with a poor response to CT. Conventionally fractionated radiation doses of up to 60 Gy showed excellent disease control in IRS-I and early single-institution studies, but enthusiasm waned as late toxicities emerged (24-26). IRS-IV randomized patients to 50.4 Gy in conventional 1.8-Gy fractions or to a hyperfractionated 59.4 Gy at 1.1 Gy per fraction given twice daily, in the hopes of reducing late toxicities and potentially improving disease control. However, the hyperfractionated arm of IRS-IV was found to be more acutely toxic and did not demonstrate an LC benefit (LF conventionally fractionated RT, 12%; hyperfractionated RT, 20%, P=.25) (27).
The lack of LC benefit with hyperfractionated dose escalation in IRS-IV may have resulted from poor compliance by younger patients requiring twice-daily anesthesia, prolongation of treatment times because of severe acute toxicity, and uncertainty regarding the α/β ratio of rhabdomyosarcoma (28). Furthermore, the dose escalation in the hyperfractionated arm of IRS-IV was modest, with a biologically effective dose (BED) of 66 Gy compared with 60 Gy for standard fractionation (assuming an α/β ratio of 10). Examination of radiation dose response in a variety of human tumor types has demonstrated that on average the doses required to control macroscopic disease are 12 Gy higher than those that control microscopic disease, and thus it is possible that the dose increase in IRS-IV was inadequate (29). In addition, the hyperfractioned arm was not selectively applied to higher-risk patients. Because 50.4 Gy is sufficient for LC in approximately 80% to 90% of group III patients, the benefit in the small group at highest risk for LF may have been obscured (1, 27, 30). In our study, 50.4 Gy was sufficient for LC in all patients with a 45% or greater reduction in tumor volume after induction CT but failed to achieve LC when the response to induction CT was ≤33%, presumably because of the large volume of residual macroscopic disease. Therefore, in this modern era when highly conformal methods such as intensity modulated RT and proton therapy allow for more precise delivery of the high-dose radiation volume, a re-examination of radiation dose escalation may be worthwhile in selected patients with poor response to induction CT.
Other options for treatment alteration in poor responders may be to modify or intensify CT regimens in the hopes of better chemical debulking, although the best cytoxic drugs are already being used in front-line therapy. In the near future, genetic profiling or other biological interrogation of a particular tumor for driver mutations and activated pathways may help better tailor therapy in the nonresponders, given that some preliminary studies seem promising (31).
As a single-institution study, our results can only be viewed as hypothesis generating. Still, the significance of these findings warrants further subset analyses in larger studies. Complementary modes of response assessment including PET–computed tomography or histologic examination may be useful in strengthening the correlation. Ultimately, if our findings are supported by larger studies, intensification of local therapy, systemic therapy, or both for poor responders and de-escalation of therapy for favorable responders may be attractive options for further exploration in future clinical trials involving patients with embryonal PM-RMS.
Summary.
In this study we demonstrate that children with parameningeal embryonal rhabdomyosarcoma and a poor response to induction chemotherapy have an increased risk of local failure.
Acknowledgments
Supported by the Federal Share of program income earned by Massachusetts General Hospital on C06 CA059267, Proton Therapy Research and Treatment Center.
Footnotes
Conflict of interest: N. J. Tarbell holds stock options (zero value) in the ProCure corporation and has an immediate family member on the ProCure board of advisors. The authors report no other conflict of interest.
References
- 1.Merks JH, De Salvo GL, Bergeron C, et al. Parameningeal rhabdomyosarcoma in pediatric age: Results of a pooled analysis from North American and European cooperative groups. Ann Oncol. 2014;25:231–236. doi: 10.1093/annonc/mdt426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spalding AC, Hawkins DS, Donaldson SS, et al. The effect of radiation timing on patients with high-risk features of parameningeal rhabdomyosarcoma: An analysis of IRS-IV and D9803. Int J Radiat Oncol Biol Phys. 2013;87:512–516. doi: 10.1016/j.ijrobp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazzoleni S, Bisogno G, Garaventa A, et al. Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer. 2005;104:183–190. doi: 10.1002/cncr.21138. [DOI] [PubMed] [Google Scholar]
- 4.Pappo AS, Anderson JR, Crist WM, et al. Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the Intergroup Rhabdomyosarcoma Study Group. J Clin Oncol. 1999;17:3487–3493. doi: 10.1200/JCO.1999.17.11.3487. [DOI] [PubMed] [Google Scholar]
- 5.Chisholm JC, Marandet J, Rey A, et al. Prognostic factors after relapse in nonmetastatic rhabdomyosarcoma: A nomogram to better define patients who can be salvaged with further therapy. J Clin Oncol. 2011;29:1319–1325. doi: 10.1200/JCO.2010.32.1984. [DOI] [PubMed] [Google Scholar]
- 6.Ferrari A, Miceli R, Meazza C, et al. Comparison of the prognostic value of assessing tumor diameter versus tumor volume at diagnosis or in response to initial chemotherapy in rhabdomyosarcoma. J Clin Oncol. 2010;28:1322–1328. doi: 10.1200/JCO.2009.25.0803. [DOI] [PubMed] [Google Scholar]
- 7.Rodeberg DA, Stoner JA, Garcia-Henriquez N, et al. Tumor volume and patient weight as predictors of outcome in children with intermediate risk rhabdomyosarcoma: A report from the Children's Oncology Group. Cancer. 2011;117:2541–2550. doi: 10.1002/cncr.25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantonello TM, Stark M, Timmermann B, et al. Tumour volume reduction after neoadjuvant chemotherapy impacts outcome in localised embryonal rhabdomyosarcoma. Pediatr Blood Cancer. 2015;62:16–23. doi: 10.1002/pbc.25207. [DOI] [PubMed] [Google Scholar]
- 9.Dharmarajan KV, Wexler LH, Gavane S, et al. Positron emission tomography (PET) evaluation after initial chemotherapy and radiation therapy predicts local control in rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2012;84:996–1002. doi: 10.1016/j.ijrobp.2012.01.077. [DOI] [PubMed] [Google Scholar]
- 10.Burke M, Anderson JR, Kao SC, et al. Assessment of response to induction therapy and its influence on 5-year failure-free survival in group III rhabdomyosarcoma: The Intergroup Rhabdomyosarcoma Study-IV experience–a report from the Soft Tissue Sarcoma Committee of the Children's Oncology Group. J Clin Oncol. 2007;25:4909–4913. doi: 10.1200/JCO.2006.10.4257. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg AR, Anderson JR, Lyden E, et al. Early response as assessed by anatomic imaging does not predict failure-free survival among patients with Group III rhabdomyosarcoma: A report from the Children's Oncology Group. Eur J Cancer. 2014;50:816–823. doi: 10.1016/j.ejca.2013.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 13.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 14.Dantonello TM, Int-Veen C, Harms D, et al. Cooperative trial CWS-91 for localized soft tissue sarcoma in children, adolescents, and young adults. J Clin Oncol. 2009;27:1446–1455. doi: 10.1200/JCO.2007.15.0466. [DOI] [PubMed] [Google Scholar]
- 15.Koscielniak E, Harms D, Henze G, et al. Results of treatment for soft tissue sarcoma in childhood and adolescence: A final report of the German Cooperative Soft Tissue Sarcoma Study CWS-86. J Clin Oncol. 1999;17:3706–3719. doi: 10.1200/JCO.1999.17.12.3706. [DOI] [PubMed] [Google Scholar]
- 16.Koscielniak E, Jurgens H, Winkler K, et al. Treatment of soft tissue sarcoma in childhood and adolescence: A report of the German Cooperative Soft Tissue Sarcoma Study. Cancer. 1992;70:2557–2567. doi: 10.1002/1097-0142(19921115)70:10<2557::aid-cncr2820701027>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Wharam MD, Hanfelt JJ, Tefft MC, et al. Radiation therapy for rhabdomyosarcoma: Local failure risk for Clinical Group III patients on Intergroup Rhabdomyosarcoma Study II. Int J Radiat Oncol Biol Phys. 1997;38:797–804. doi: 10.1016/s0360-3016(97)00120-x. [DOI] [PubMed] [Google Scholar]
- 18.Ladra MM, Szymonifka JD, Mahajan A, et al. Preliminary results of a phase II trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32:3762–3770. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macdonald SM, Sethi R, Lavally B, et al. Proton radiotherapy for pediatric central nervous system ependymoma: Clinical outcomes for 70 patients. Neuro Oncol. 2013;15:1552–1559. doi: 10.1093/neuonc/not121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rombi B, DeLaney TF, MacDonald SM, et al. Proton radiotherapy for pediatric Ewing's sarcoma: Initial clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1142–1148. doi: 10.1016/j.ijrobp.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 21.Sethi RV, Giantsoudi D, Raiford M, et al. Patterns of failure after proton therapy in medulloblastoma; linear energy transfer distributions and relative biological effectiveness associations for relapses. Int J Radiat Oncol Biol Phys. 2014;88:655–663. doi: 10.1016/j.ijrobp.2013.11.239. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez RB, Sethi R, Depauw N, et al. Proton radiation therapy for pediatric medulloblastoma and supratentorial primitive neuroectodermal tumors: Outcomes for very young children treated with upfront chemotherapy. Int J Radiat Oncol Biol Phys. 2013;87:120–126. doi: 10.1016/j.ijrobp.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Greenberger BA, Pulsifer MB, Ebb DH, et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int J Radiat Oncol Biol Phys. 2014;89:1060–1068. doi: 10.1016/j.ijrobp.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 24.Jereb B, Ghavimi F, Exelby P, et al. Local control of embryonal rhabdomyosarcoma in children by radiation therapy when combined with chemotherapy. Int J Radiat Oncol Biol Phys. 1980;6:827–833. doi: 10.1016/0360-3016(80)90319-3. [DOI] [PubMed] [Google Scholar]
- 25.Berry MP, Jenkin RD. Parameningeal rhabdomyosarcoma in the young. Cancer. 1981;48:281–288. doi: 10.1002/1097-0142(19810715)48:2<281::aid-cncr2820480212>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Donaldson SS, Castro JR, Wilbur JR, et al. Rhabdomyosarcoma of head and neck in children: Combination treatment by surgery, irradiation, and chemotherapy. Cancer. 1973;31:26–35. doi: 10.1002/1097-0142(197301)31:1<26::aid-cncr2820310105>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 27.Donaldson SS, Meza J, Breneman JC, et al. Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma–A report from the IRSG. Int J Radiat Oncol Biol Phys. 2001;51:718–728. doi: 10.1016/s0360-3016(01)01709-6. [DOI] [PubMed] [Google Scholar]
- 28.Timmerman RD, Mendonca M. In regard to Donaldson et al: Results from the IRS-IV randomized trial of hyperfractionated radiotherapy in children with rhabdomyosarcoma–A report from the IRSG. IJROBP 2001;51:718-728. Int J Radiat Oncol Biol Phys. 2002;54:1579–1580. doi: 10.1016/s0360-3016(02)03015-8. author reply 1580. [DOI] [PubMed] [Google Scholar]
- 29.Okunieff P, Morgan D, Niemierko A, et al. Radiation dose-response of human tumors. Int J Radiat Oncol Biol Phys. 1995;32:1227–1237. doi: 10.1016/0360-3016(94)00475-z. [DOI] [PubMed] [Google Scholar]
- 30.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: Children's Oncology Group study D9803. J Clin Oncol. 2009;27:5182–5188. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marampon F, Gravina GL, Di Rocco A, et al. MEK/ERK inhibitor U0126 increases the radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by downregulating growth and DNA repair signals. Mol Cancer Ther. 2011;10:159–168. doi: 10.1158/1535-7163.MCT-10-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]