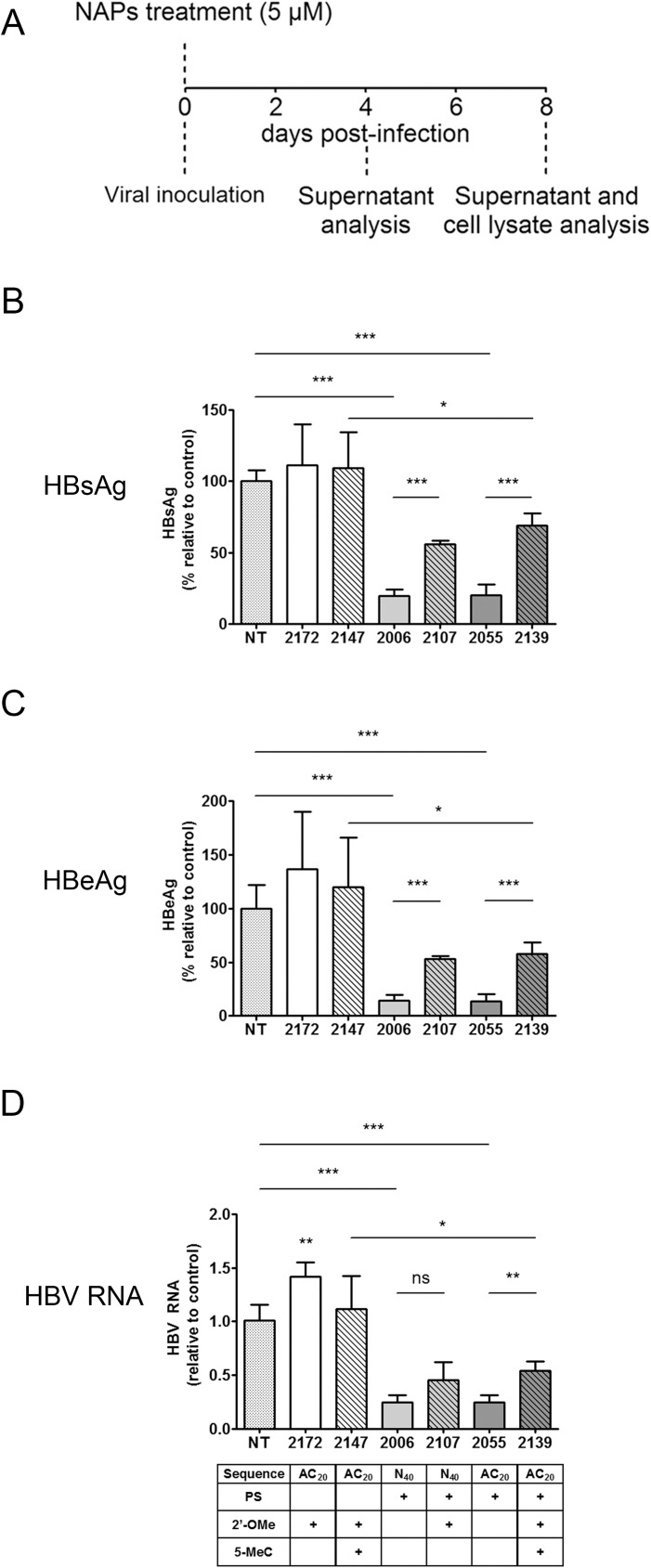
Fig 4. Relationship between NAPs sequence, amphipathicity, chemical modification and anti-HBV antiviral activity.
(A) Treatments procedure: HBV infected HepaRG cells were treated at the time of inoculation for the duration of inoculation. Supernatants and cell lysates were harvested for extracellular HBsAg, HBeAg and intracellular total HBV RNA analysis at day 4 (data not shown) and day 8 post-inoculation. (B) To determine the effect of different NAPs chemical modifications on antiviral activity, HepaRG cells were treated once at the time of viral inoculation with REP 2172, REP 2147, REP 2006, REP 2107, REP 2055 and REP 2139 NAP compounds containing or not PS (phosphorothioation), 2’-OMe (2’O-methyl ribose) and 5-MeC (5-methylcytidine) at a 5 μM concentration and secreted HBsAg, HBeAg and total HBV RNA were measured at day 8 post-inoculation. The solvent of NAP compounds was used as a non-treated condition. All data originating from two independent experiments are expressed as means ± standard deviation. Statistical analysis was conducted with unpaired, 2-tailed t-tests, for comparison of specific samples; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001, ns; non-significant.