Figure 9.
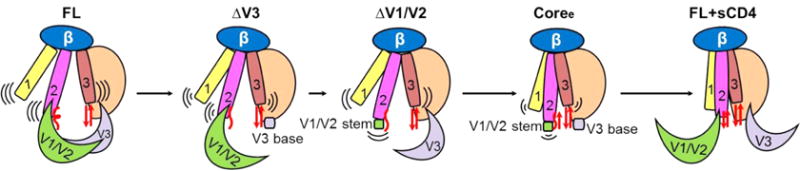
Model of conformational and dynamic changes in the unliganded gp120 monomer upon deletion of V1/V2 and V3 loops. The unliganded wild-type full-length gp120 (FL) (left) had the highest dynamics in three layers of the inner domain with unfolded bridging sheets and masked immature coreceptor-binding site.96 Upon deletion of the V1/V2 and V3 loops, the three layers of the inner domain became less dynamic and more ordered. Coree without all V1/V2 and V3 loops showed more stability toward the CD4-bound state with an ordered inner domain and well-folded bridging sheets. The CD4 engagement (right) induced a fixed, stable conformation with formation of the bridging sheets and movement of V1/V2 loops to expose a mature coreceptor-binding site on the bridging sheet and V3 loop.
