Fig. 4. Electrochemical behaviors of amorphous BSCF nanofilms on Ni foam substrates.
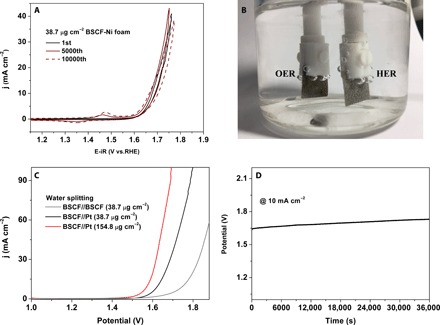
(A) Current-potential profile at the 1st, 5000th, and 10,000th cycles for the 38.7 μg cm−2 BSCF–Ni foam heterostructure electrode in a 0.1 M KOH solution. (B) Two-electrode electrolyzer setup consisting of 38.7 μg cm−2 BSCF–Ni foam anode and 38.7 μg cm−2 Pt–Ni foam cathode in a 1.0 M KOH solution. (C) Typical current-potential profiles from the two-electrode electrolyzer during water splitting. (D) Time-dependent potential response during 10-hour chronoamperometric water-splitting reaction using the two-electrode electrolyzer (38.7 μg cm−2 BSCF–Ni foam anode and 38.7 μg cm−2 Pt–Ni foam cathode) at a constant current density of 10 mA cm−2.
