Abstract
We recently isolated a tumoricidal peptide from Natto, a Japanese traditional fermented food. In the present study, antimicrobial activity of the Natto peptide was examined. The peptide consisted of 45 amino acid residues, and its structure was predicted to be rich in α-helix. It excreted antimicrobial activity only against Streptococcus pneumoniae and Bacillus subtilis group (B. subtilis, Bacillus pumilus, and Bacillus licheniformis). Lesser antimicrobial activity was observed for Streptococcus species other than S. pneumoniae. Hemolysate or hemin was required for the antimicrobial activity of the peptide. The Natto peptide damages the cell membrane of B. subtilis. On the other hand, chain morphology was induced in S. pneumoniae, which is naturally diplococcus, during the early phases of the Natto peptide treatment; following that the cells were rapidly lysed. This suggested that the Natto peptide displayed a novel narrow spectrum of bactericidal activity and inhibited cell separation during cell division of S. pneumoniae.
Keywords: Natto, Antimicrobial peptide, Streptococcus pneumoniae, Bacillus subtilis, Subtilisin
Introduction
Natto is a traditional Japanese food obtained after fermentation of boiled soybeans by Bacillus subtilis. It is recognized as a healthy food, and a rich source of vitamins, calcium, and various nutrients. Isoflavone, an analogue of estrogen, is derived from soybeans, and it is shown to prevent cerebral and myocardial stroke, breast cancer and prostate cancer (Zaheer and Humayoun Akhtar 2017). Lecithin is also derived from soybeans; it is a phospholipid containing unsaturated fatty acids. Lecithin has shown to prevent coronary artery diseases by preventing the deposition of cholesterol and other fats on the vascular endothelium and to prevent liver damages (Nicolosi et al. 2001). Nattokinase is a subtilisin family serine protease derived from B. subtilis. It exhibits a pronounced fibrinolytic activity, and is known as a blood thinner (Dabbagh et al. 2014; Sumi et al. 1990). Natto is the only food containing high levels of water-soluble vitamin K2 (menaquinone-7). The high vitamin K2 content of Natto results from fermentation; consequently, vitamin K2 content in Natto is over 100 times higher than in soybeans (Sakano et al. 1988). Natto, therefore, contains numerous useful substrates for human health derived from the soybeans, B. subtilis, and soybean fermentation products. We were interested in the new beneficial effects of the multifunctional food, Natto, on human health.
Recently, we discovered a new peptide (termed “Natto peptide”) that has cytotoxicity toward tumor cell lines but not toward normal cell lines (Hatakeyama et al. 2016). Injury of the cell membrane was suggested as a mechanism of this cytotoxicity. Such tumoricidal activity is also displayed by various antimicrobial peptides. One family of such antimicrobial peptides is the defensin family (Mattar et al. 2016; Suarez-Carmona et al. 2015). The defensin family peptides are disulfide-bond-rich cationic peptides found in both vertebrates and invertebrates. In human, α-defensins are mainly produced by neutrophils, and β-defensins are produced by leukocytes and epithelial cells. Another antimicrobial peptide is the cathelicidin family (Wang et al. 2014; Xhindoli et al. 2016). They are α-helix-rich peptides, with basic amino acids present every few residues. They are found in mammals, and are produced by neutrophils, macrophages, and keratinocytes. These cationic peptides display tumoricidal activity like as the Natto peptide and antimicrobial activity against various types of microorganisms, including Gram-positive bacteria, Gram-negative bacteria, and fungi (Mattar et al. 2016; Sang and Blecha 2008; Suarez-Carmona et al. 2015; Xhindoli et al. 2016). Given the above, we were interested in the antimicrobial activity of the Natto peptide.
Materials and methods
Preparation of the Natto peptide
Natto, which is commercially available, was donated by Yamada Foods (Misato, Akita Prefecture, Japan). Six Natto preparations were obtained, generated by using different B. subtilis strains. Natto was homogenized with Polytron PT4000 (Kinematica AG, Luzern, Switzerland) and centrifuged at 20,000×g for 15 min at 4 °C. Saturated ammonium sulfate was added to the supernatant at a concentration of 30%, and the mixture was stirred for 30 min at 4 °C and centrifuged at 20,000×g for 15 min at 4 °C. The supernatant was collected, and then saturated ammonium sulfate was added at a concentration of 50%. The mixture was stirred for 30 min at 4 °C and centrifuged at 20,000×g for 15 min at 4 °C. The resulting precipitate (30–50% saturated ammonium sulfate precipitated fraction) was dialyzed against 10 mM Tris–HCl (pH 7.4) for 24 h. The resulting material was precipitated with 25% saturated ammonium sulfate at 4 °C, and the supernatant was filtered through a 0.45 μm membrane filter. The filtrate was applied to a column of Butyl-Sepharose High Performance (GE Healthcare Life Sciences, Chalfont St Giles, UK) equilibrated with 10 mM Tris–HCl (pH 7.4) containing 25% saturated ammonium sulfate. The column was successively eluted with 25, 20, 15, 10, 5 and 2% saturated ammonium sulfate in 10 mM Tris–HCl (pH 7.4). The eluent with 10% saturated ammonium sulfate was dialyzed in 10 mM Tris–HCl (pH 7.4), lyophilized, and used as a Natto peptide preparation. The Natto peptide was yielded approximately 1 g from 500 g of Natto.
The purity of the peptide was confirmed by Tris/Tricine-sodium dodecyl sulfate–polyacrylamide gel electrophoresis (Tris/Tricine-SDS-PAGE) (Schägger and von Jagow 1987) and Coomassie Brilliant Blue (CBB) staining.
The protein concentration was determined by the method of Bradford (1976) using bovine serum albumin as a standard.
Amino acid sequence of the Natto peptide
For amino acid sequence determination, the Natto peptide preparation was further purified by column chromatography on Wakosil-5C18HG column (Wako Pure Chemical, Tokyo, Japan). The peptide was eluted with acetonitrile [linear gradient of 0–60% (v/v)]. The fraction eluted with approximately 30% acetonitrile was pooled and lyophilized. The resulting material was analyzed. Amino acid sequence of the peptide was determined using the protein sequencer PPSQ-31B/33B (Shimadzu, Kyoto, Japan).
Bacterial strains
Bacillus subtilis, AHU 1035, AHU 1037, AHU 1604, AHU 1615, AHU 1708T and AHU 1722, Bacillus cereus AHU 1358, Bacillus licheniformis AHU 1371, Bacillus megaterium AHU 1373, and Bacillus pumilus AHU 1386 were obtained from the Research Faculty of Agriculture, Hokkaido University (Sapporo, Japan). B. cereus JCM 2152T, Streptococcus agalactiae JCM 5671T, Streptococcus mitis JCM 12971T, Streptococcus mutans JCM 5705T, Streptococcus pyogenes JCM 5674T, Streptococcus salivarius JCM 5707T, and Streptococcus sanguinis JCM 5708T were obtained from the Japan Collection of Microorganisms, Riken BioResource Center (Tsukuba, Japan). Acinetobacter baumannii ATCC 19606T, Escherichia coli ATCC 25922 and ATCC 35218, Lactobacillus fermentum ATCC 9338, Lactobacillus rhamnosus ATCC 7469T, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923, Streptococcus pneumoniae ATCC 49619, and Haemophilus influenzae ATCC 51907 were obtained from the American Type Culture Collection (Manassas, VA, USA). Lactobacillus plantarum NRIC 1067T was obtained from the NODAI Culture Collection Center, Tokyo University of Agriculture (Tokyo, Japan). A. baumannii SRAC2, S. aureus SUNA1 [hospital-acquired methicillin-resistant S. aureus (MRSA)] and SR-581 (community-acquired MRSA), Enterococcus faecium M2483, Enterococcus faecalis HU1 [vancomycin-resistant Enterococcus (VRE)] and M2486, E. coli 7249 (ST131), P, aeruginosa PA103 and 9728 (mucoid), Serattia marcescens SRSM2, S. pneumoniae SR11, MH101 [penicillin-resistant S. pneumoniae (PRSP)], 237 (PRSP) and 442 (mucoid), and Candida albicans SRCA1 and SRCA4 were clinical isolates stocked in our laboratory. S. pneumoniae R6 and P103 [lytA-deficient mutant derived from R6 (ΔlytA)] were kindly provided by Dr. Ernesto García (Centro de Investigaciones Biológicas, CSIC, Madrid, Spain) (Moscoso et al. 2014).
Minimum inhibitory concentration
Antimicrobial activity was estimated by minimum inhibitory concentration (MIC) determinations using a broth microdilution procedure basically according to a guideline of the Clinical Laboratory Standards Institute (14). Briefly, cell suspensions (5 × 105 cells/mL) and serially diluted solutions of the Natto peptide were mixed in a 96 well microplate, and cultured at 37 °C for 24 h. The basal medium was Mueller-Hinton (MH) broth (Becton–Dickinson, Franklin Lakes, NJ, USA) or Todd Hewitt (TH) broth (Becton–Dickinson) supplemented with 5% (v/v) horse hemolysate (Nippon Bio-Test Laboratories, Tokyo, Japan).
The combined effect of the Natto peptide and other antibacterial agents
Cefditoren and tebipenem were provided by Meiji Seika Pharma (Tokyo, Japan). Clarithromycin was provided by Taisho Pharmaceutical (Tokyo, Japan). Amoxicillin, amikacin, tobramycin, and gentamicin were purchased from Wako Pure Chemical. Levofloxacin was purchased from LKT Laboratories (St. Paul, MN, USA). A combined effect was defined according to the fractional inhibitory concentration (FIC) index deduced from MIC values, as follows: synergistic effect, FIC ≤ 0.5; additive effect, FIC 0.6–1.0; no effect, FIC 1.1–2; and antagonistic effect, FIC > 2.
The effect of supplements on the antimicrobial activity of the Natto peptide
Hemin was purchased from Wako Pure Chemical. Horse serum was from Thermo Fisher Scientific (Waltham, MA, USA). Heat-treated horse hemolysate was prepared by heating at 100 °C for 10 min; various concentrations were suspended in TH broth, and then centrifuged at 9000×g for 5 min. The supernatant was filtered (0.22 µm) and used in culture media for MIC measurements. The effect on antimicrobial activity was determined by MIC measurements using the broth microdilution method. Briefly, suspensions of S. pneumoniae R6 cells (5 × 105 cells/mL) in TH broth containing serial dilutions of the Natto peptide and serial dilutions of the supplements were cultured at 37 °C for 24 h.
The effect of the Natto peptide on bacterial cell growth and survival
Streptococcus pneumoniae and B. subtilis cells were precultured on TH agar containing 5% (v/v) horse hemolysate at 37 °C for 12 h. The cells were then suspended in saline, and used to inoculate TH broth containing 5% (v/v) horse hemolysate at an optical density at 625 nm of 0.000625 (approximately 1 × 105 cells/mL). The Natto peptide was added to the cell cultures at the time of inoculation or 4 h after the inoculation (approximately 1 × 107 cells/mL). Cell numbers were determined by plating aliquots of the cultures sampled at various times on TH agar containing 5% (v/v) horse hemolysate, and culturing under microaerophilic conditions; the resulting colonies were then counted.
Morphological observations of the Natto peptide treated cells
Streptococcus pneumoniae and B. subtilis cells (control cells or cells treated with the Natto peptide) were observed by transmission electron microscopy (TEM) after negative staining as previously described (O’Connell Motherway et al. 2011), with minor modifications. The bacterial cells were cultured in TH broth containing 5% (v/v) horse hemolysate at 37 °C for 4 or 8 h in the presence or absence of the Natto peptide. Cells were fixed with 1% (v/v) glutaraldehyde for 30 min at 4 °C. Copper grids (150 mesh; Veco B.V., Eerbeek, the Netherlands) were floated on the fixed cell suspensions for 10 min at room temperature. The grids were washed once for 5 min in 0.02 M glycine-phosphate-buffered saline, and three times for 5 min in ultra-pure water. Negative staining was performed using an EM stainer (Nisshin EM, Tokyo, Japan). The grids were observed using JEOL JEM-1400 TEM (JEOL, Tokyo, Japan) operated at 80 kV.
Results
Characterization of the Natto peptide
The Natto peptide was successfully isolated from the active fraction of homogenate of Natto by successive ammonium sulfate precipitation steps and hydrophobic interaction chromatography (Hatakeyama et al. 2016). The resulting peptide migrated as a single band of approximately 5 kDa, as estimated by Tris/Tricine-SDS-PAGE and CBB staining (Fig. 1). The Natto peptide consisted of 45 amino acid residues and its sequence was shown in Fig. 2. It shared extremely high homology with a sequence near the C-terminal side of subtilisin family proteins, which are serine proteases produced by Bacillus species. High, 97.8%, homology with nattokinase of B. subtilis (GenBank ACJ48969.1) (Fig. 2) and 80.0% homology with keratinase of B. licheniformis (GenBank AID16241.1) were observed.
Fig. 1.
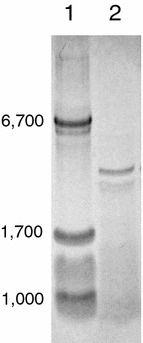
Tirs/Tricine-SDS-PAGE analysis of the Natto peptide preparation. The sample was applied on a 15–20% gradient polyacrylamide gel. Lane 1 molecular weight standards. Lane 2 Natto peptide preparation at 10 μg/lane
Fig. 2.
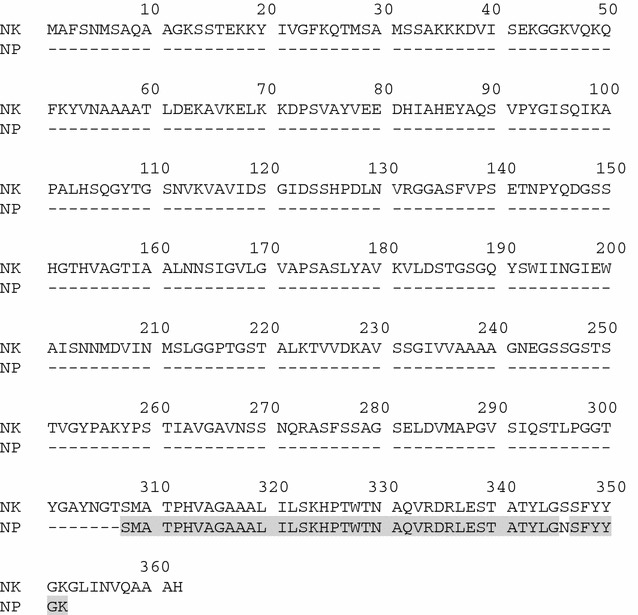
Comparison of amino acid sequence of nattokinase (NK) and Natto peptide (NP). The amino acid sequence of NK is cited from ACJ48969.1
Antimicrobial activity of the Natto peptide
The antimicrobial activity of the Natto peptide was determined by MIC measurements with various microorganisms. The MH medium was first used in the assays, according to the CLSI recommendations. The Natto peptide showed antimicrobial activity only S. pneumoniae (data not shown). MIC for S. pneumoniae was determined using MH broth supplemented with 5% (v/v) hemolysate (14). Next, MIC values were determined using TH broth, a medium more rich than MH broth, supplemented with 5% (v/v) horse hemolysate (Table 1). The Natto peptide displayed antimicrobial activity against S. pneumoniae, including PRSP and mucoid strains, and B. subtilis, B. pumilus, and B. licheniformis, at a concentration of 8 or 16 μg/mL. Lesser activity was observed against strains of Streptococcus species other than S. pneumoniae and against L. fermentum at a concentration of 64 or 128 μg/mL. The Natto peptide did not display any antimicrobial activity against other Gram-positive bacteria, including B. cereus and B. megaterium, any of the Gram-negative bacteria examined, and C. albicans.
Table 1.
MICs of the Natto-peptide tested against various Gram-positive bacteria, Gram-negative bacteria, and fungi
| Strain | MIC (μg/mL) |
|---|---|
| Gram-positive bacteria | |
| B. cereus JCM 2152T | >128 |
| B. cereus AHU 1358 | >128 |
| B. licheniformis AHU 1371 | 8 |
| B. megaterium AHU 1373 | >128 |
| B. pumilus AHU 1386 | 8 |
| B. subtilis AHU 1708T | 8 |
| B. subtilis AHU 1035 | 16 |
| B. subtilis AHU 1037 | 8 |
| B. subtilis AHU 1604 | 8 |
| B. subtilis AHU 1615 | 16 |
| B. subtilis AHU 1722 | 16 |
| E. faecalis HU1 (VRE) | >128 |
| E. faecalis M2486 | >128 |
| E. faecium M2483 | >128 |
| L. fermentum ATCC 9338 | 64 |
| L. plantarum NRIC 1067T | >128 |
| L. rhamnosus ATCC 7469T | >128 |
| S. aureus ATCC 25923 | >128 |
| S. aureus SUNA1 (HA-MRSA) | >128 |
| S. aureus SR-581(CA-MRSA) | >128 |
| S. agalactiae JCM 5671T | 64 |
| S. mitis JCM 12971T | 64 |
| S. mutans JCM 5705T | 64 |
| S. pneumoniae ATCC 49619 | 8 |
| S. pneumoniae R6 | 8 |
| S. pneumoniae P103 (ΔlytA) | 8 |
| S. pneumoniae SR11 | 16 |
| S. pneumoniae MH101 (PRSP) | 16 |
| S. pneumoniae 237 (PRSP) | 8 |
| S. pneumoniae 442 (mucoid) | 8 |
| S. pyogenes JCM 5674T | 128 |
| S. salivarius JCM 5707T | 128 |
| S. sanguinis JCM 5708T | 64 |
| Gram-negative bacteria | |
| A. baumannii ATCC 19606T | >128 |
| A. baumannii SRAC2 | >128 |
| E. coli ATCC 25922 | >128 |
| E. coli ATCC 35218 | >128 |
| E. coli 7249 (ST131) | >128 |
| H. influenza ATCC 51907 | >128 |
| P. aeruginosa ATCC 27853 | >128 |
| P. aeruginosa PA103 | >128 |
| P. aeruginosa 9728 (mucoid) | >128 |
| S. marcescens SRSM2 | >128 |
| Fungi | |
| C. albicans SRCA1 | >128 |
| C. albicans SRCA4 | >128 |
Protein concentrations were adjusted by the results of colorimetric assay as described in “Materials and methods”
Natto peptides obtained from various Natto preparations that were prepared using different soybeans and different B. subtilis strains also displayed specific antimicrobial activity against S. pneumoniae and B. subtilis, however, the activities were different in some instances (Table 2). This indicated that the Natto peptide was typically and commonly present in Natto.
Table 2.
Antimicrobial activity of the Natto peptide from various Natto preparations obtained using different soybeans and B. subtilis strains
| Natto preparation lot No. | B. subtilis strain used for starter | MIC (μg/mL) | |
|---|---|---|---|
| S. pneumoniae R6 | B. subtilis AHU 1708T | ||
| 160 | S63 | 64 | 128 |
| 170 | H23 | 8 | 16 |
| 180 | S125 | 8 | 16 |
| 200 | S278 | 8 | 8 |
| 300 | S125-5 | 32 | 32 |
The combined effect of the Natto peptide and other antimicrobial agents
Next, the combined effect of the Natto peptide and antimicrobial agents was examined. The tested antimicrobials were aminoglycosides (that injure the cell membrane in addition to inhibition of protein synthesis) and drugs frequently used in pneumococcal infections. The antimicrobial agents examined did not affect (synergistically, additively or antagonistically) to the Natto prptide activity, as assessed by the FIC index analysis (Table 3).
Table 3.
Combined antimicrobial effect (FIC index) of the Natto peptide and other antimicrobial agents
| Antibiotic | FIC index |
|---|---|
| Amoxicillin | 2 |
| Cefditoren | 2 |
| Tebipenem | 2 |
| Levofloxacin | 2 |
| Clarithromycin | 1.38 |
| Amikacin | 1.09 |
| Tobramycin | 2 |
| Gentamicin | 2 |
Hemin is required for antimicrobial activity of the the Natto peptide
Horse hemolysate was required for the occurrence of antimicrobial activity of the Natto peptide in a dose-dependent manner (Table 4). Heat treatment (100 °C, 10 min) of horse hemolysate resulted in the aggregation of many proteins present in the hemolysate. The aggregates were removed by centrifugation and filtration. The resulting supernatant retained the ability to support the antimicrobial activity of the Natto peptide. This suggested that heat-stable substance(s) present in the horse hemolysate were required for the antimicrobial activity of the peptide. Horse serum did not alter the antimicrobial activity of the peptide, therefore, blood cell component(s) were subsequently evaluated. Hemin is used as a supplement in MIC measurements with H. influenzae as the X factor (White and Granick 1963). The antimicrobial activity of the Natto peptide against S. pneumoniae was supported by hemin in a concentration-dependent manner (Table 4). The amount of hemin required for the occurrence of the antimicrobial activity was comparable with the amount of heme (5–6 mg/L) in horse hemolysate required for the occurrence of the antimicrobial activity.
Table 4.
The effect of medium supplemented with horse hemolysate, hose serum, and hemin on the MIC of the Natto peptide with S. pneumoniae R6 strain
| Supplement | Concentrationb | MIC (μg/mL) |
|---|---|---|
| None | – | >128 |
| Horse hemolysatea | 5% | 8 |
| 1% | 4 | |
| 0.32% | 4 | |
| 0.10% | 8 | |
| 0.032% | 32 | |
| 0.010% | 64 | |
| 0.0032% | 128 | |
| 0.0010% | >128 | |
| Supernatant of heat-treated horse hemolysate | 20% | 4 |
| 5% | 32 | |
| Horse serum | 5% | >128 |
| Hemina | 6.9 μg/mL | 8 |
| 3.4 μg/mL | 32 | |
| 1.7 μg/mL | 64 | |
| 0.86 μg/mL | 128 | |
| 0.43 μg/mL | >128 |
aHose hemolysate [5%(v/v)] and hemin (6.9 μg/mL) did not any antimicrobial effects against S. pneumoniae
bThe percent concentrations are (v/v)
The effect of the Natto peptide on bacterial cell growth and survival
Survival curves of S. pneumoniae and B. subtilis cell cultures in the presence or absence of the Natto peptide were examined. The growth of S. pneumoniae was similar during the early phases of growth in the presence and absence of the Natto peptide (Fig. 3). When high cell numbers were used in the inoculum (approximately 107 cells/mL), the cultures reached maximum cell density (approximately 108 cells/mL) regardless of the presence of the Natto peptide; the number of viable cells then decreased (Fig. 3a). The rate of the decrease of viable cell numbers was markedly higher in the presence of the Natto peptide than in the absence of the peptide. When low cell numbers were used in the inoculum (approximately 105 cells/mL), in the absence of the peptide, the culture reached maximum cell density (approximately 108 cells/mL) after 8 h, following which the viable cell numbers decreased. On the other hand, in the presence of the peptide, the culture did not reach maximum cell numbers and the number of viable cells rapidly decreased 2 h after the addition of Natto peptide (Fig. 3b). These results suggested that S. pneumoniae cells initially grew normally in the presence of the Natto peptide; the growth was then terminated and the cells rapidly lysed. The rapid decrease of viable S. pneumoniae cells seemed to be associated with autolysis. Consequently, the contribution of LytA, the main S. pneumoniae-specific autolysin, to the decrease of viable cells in late phase was examined, using the lytA-deficient mutant P103. The rate of viable cell number decrease in the presence of the Natto peptide was lower in P103 than in the parent strain R6 (Fig. 3b, c). This indicated that the autolysis caused by LytA enhanced the bactericidal rate, but it was not essential for the antimicrobial activity of the Natto peptide.
Fig. 3.
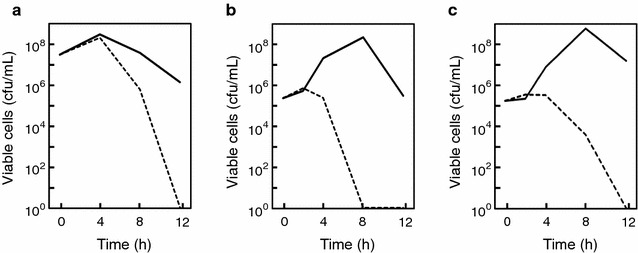
Survival curves of S. pneumoniae strains in the presence and absence of the Natto peptide. a S. pneumoniae R6 cells were pre-cultured to a cell density of approximately 107 cells/mL in TH broth containing 5% (v/v) horse hemolysate. The Natto peptide was then added at a concentration of 64 μg/mL. b, c S. pneumoniae R6 (b) and P103 (lytA-deficient mutant derived from R6) (c) cells were suspended at a density of approximately 105 cells/mL in TH broth containing 5% (v/v) horse hemolysate. The Natto peptide was then added at a concentration of 64 μg/mL. Throughout the cultivation, the cultures were sampled, and culture aliquots were spread on a TH agar supplemented with 5% (v/v) horse hemolysate, and cultured for 24 h. Grown colonies were then counted. Dashed lines indicate cell numbers in the presence of the Natto peptide. Solid lines indicate cell numbers in the absence of the Natto peptide. Each experiment performed at least three times, and the representative results are presented. The similar results were observed in the same experiments
The survival curves of B. subtilis cells also indicated that viable cell numbers rapidly decreased in the presence of the Natto peptide (Fig. 4). The decrease occurred immediately after the addition of the Natto peptide, and seen when both high (approximately 107 cells/mL; Fig. 4a) and low (approximately 105 cells/mL; Fig. 4b) inocula were used. This suggested that the antibacterial action of the Natto peptide differed in S. pneumoniae and B. subtilis.
Fig. 4.
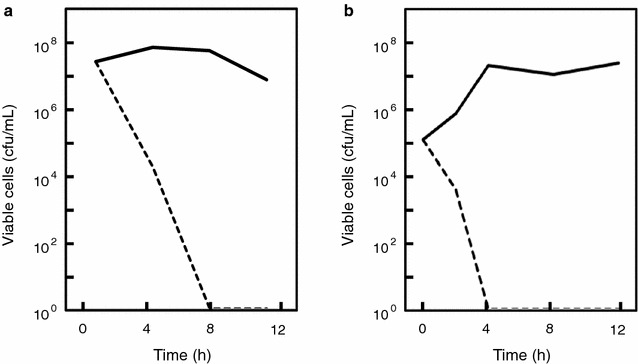
Survival curves of B. subtilis strain in the presence and absence of the Natto peptide. a B. subtilis AHU 1708T cells were pre-cultured to a cell density of approximately 107 cells/mL in TH broth containing 5% (v/v) horse hemolysate. The Natto peptide was then added at a concentration of 64 μg/mL. b B. subtilis AHU 1708T cells were suspended at a cell density of approximately 105 cells/mL in TH broth containing 5% (v/v) horse hemolysate. The Natto peptide was then added at a concentration of 64 μg/mL, and the cells were cultured. Throughout the cultivation, the cultures were sampled, and culture aliquots were spread on TH agar supplemented with 5% (v/v) horse hemolysate, and cultured for 24 h. Grown colonies were then counted. Dashed lines indicate cell numbers in the presence of the Natto peptide. Solid lines indicate cell numbers in the absence of the Natto peptide. Each experiment performed at least three times, and the representative results are presented. The similar results were observed in the same experiments
Morphological observations of cells treated with the Natto peptide
Streptococcus pneumoniae cell morphology was examined by TEM after negative staining (Fig. 5). In the absence of the Natto peptide, S. pneumoniae cells looked like typical diplococci (Fig. 5a). The cells formed chain-like structure 4 h after the addition of the Natto peptide (Fig. 5b). These results suggested that the cells failed to separate after cell division in the presence of the peptide. After 8 h of culture, the observed cell lysis was more pronounced in the presence than the absence of the Natto peptide (Fig. 5c, d).
Fig. 5.
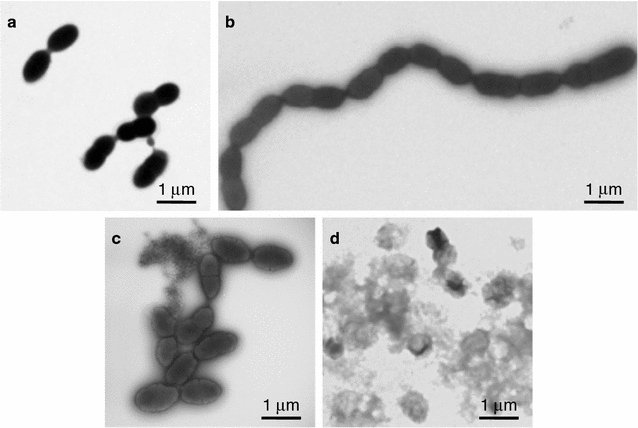
TEM analysis with negative staining of S. pneumoniae R6 cells cultured in the presence or absence of the Natto peptide. The cells were cultured in TH broth containing 5% (v/v) horse hemolysate in the absence (a, c) or presence (b, d) of the Natto peptide (64 μg/mL). After 4 h (a, b) or 8 h (c, d) of incubation, the cells were harvested and observed TEM after negative staining
In the case of B. subtilis (Fig. 6), localized cell membrane damages and cell swelling were observed 4 h after that Natto peptide addition (Fig. 6b). After 8 h when no viable cells were seen, the cells completely lysed (Fig. 6d).
Fig. 6.
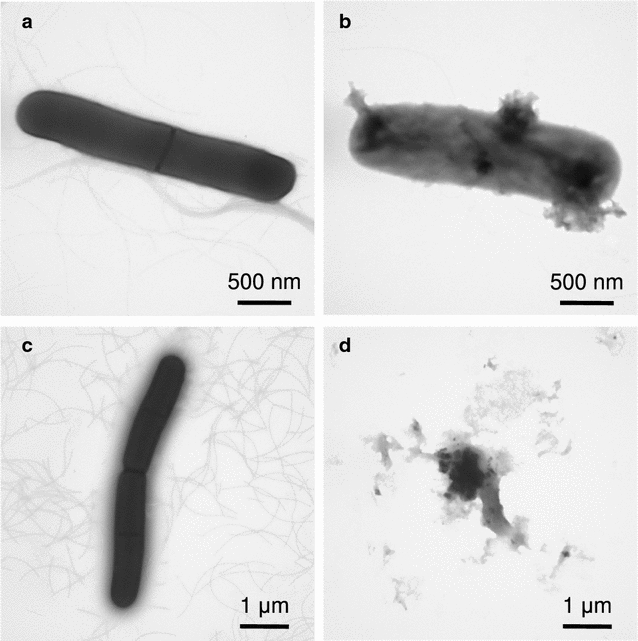
TEM analysis with negative staining of B. subtilis AHU 1708T cells cultured in the presence or absence of the Natto peptide. The cells were cultured in TH broth containing 5% (v/v) hemolysate in the absence (a, c) or presence (b, d) of the Natto peptide (64 μg/mL). After 4 h (a, b) or 8 h (c, d) of incubation, the cells were harvested and observed using TEM after negative staining
Discussion
Recently, we reported a tumoricidal activity of the Natto extract; approximately 5 kDa peptide was identified as the active component (Hatakeyama et al. 2016). As shown here, the peptide consisted of 45 amino acid residues, and shared extremely high homology with the C-terminal region of a subtilisin family serine protease, Nattokinase produced by B. subtilis. The structure of the peptide is probably rich in α-helix deduced from secondary structure predictions and the presence of basic amino acid residues appearing every few residues. Such structural characteristics are found in several types of antimicrobial peptides (Yount et al. 2006). Typically, the characters were found in cathelicidin family antimicrobial peptides (Wang et al. 2014; Xhindoli et al. 2016). The cathelicidin antimicrobial peptides generally have broad spectrum of antimicrobial activity and are effective against multidrug-resistant bacteria (Sang and Blecha 2008; Zanetti et al. 2002). They also show cytotoxicity toward tumor cell lines, but not toward normal cell lines, similarly to the Natto peptide. Thus, we anticipated that the Natto peptide possesses a broad-spectrum antimicrobial activity with broad spectrum, similarly to the antimicrobial peptides.
Unexpectedly, we found a novel antimicrobial activity of the Natto peptide. The spectrum of activity was very narrow, namely, only against S. pneumoniae and several Bacillus strains (Table 1). Little antimicrobial activity was observed against Streptococcus spp., including group A (S. pyogenes) and group B (S. agalactiae) streptococci and oral viridans streptococci. The Natto peptide seemed to potentially possess an antimicrobial activity against streptococci, and most pronouncedly against S. pneumoniae. On the other hand, the Natto peptide showed antimicrobial activity against B. subtilis, B. licheniformis, and B. pumilus, no antimicrobial activity was observed for B. cereus and B. megaterium. B. subtilis, B. pumilus, and B. licheniformis are phylogenetically classified into the B. subtilis group (Bhandari et al. 2013; Turenne et al. 2015); B. cereus and B. megaterium are phylogenetically distant from the B. subtilis group. The results indicated that the antimicrobial activity of the Natto peptide was specific for S. pneumoniae and the B. subtilis group of the genus Bacillus.
The antimicrobial activity was commonly observed various Natto preparations produced by different strains of B. subtilis (Table 2), whereas levels of the activity was different each other. This could be due to differences of expression levels and/or amino acid sequences of the peptides; however, this is a future issue.
The specific antimicrobial action point(s) of the Natto peptide is very interesting, i.e., the early growth phase after the addition of the peptide. Comparable S. pneumoniae cell growth was observed at early time points, regardless of whether the peptide was added. The S. pneumoniae cells formed long chains in the presence of the Natto peptide, although S. pneumoniae is naturally diplococcus as seen in the absence of the peptide (Fig. 5). The results suggested that the Natto peptide caused a failure of cell separation after cell division. At late growth phases, viable cell numbers rapidly decreased in the presence of the peptide (Fig. 3). We proposed that the cells were dead by autolysis. The rapid and pronounced cell death was attributable to LytA, which is a major autolysin specific for S. pneumoniae (Maestro and Sanz 2016); however, LytA was not a direct target of the anti-S. pneumoniae bactericidal activity of the Natto peptide. Similar long chain morphology of S. pneumoniae is found in cells treated with choline (Maestro et al. 2007). Choline interacts with several pneumococcal proteins and affects autolysis and cell division/separation (Maestro and Sanz 2016). Chain morphology is also observed in a defective mutant of LytB, a peptidoglycan hydrolase contributing to cell separation (Bai et al. 2014; De Las Rivas et al. 2002).
The current results suggest that the Natto peptide might be a fragment of subtilisin family protein, namely Nattokinase. It might be generated by a proteolytic cleavage; however, the responsible protease was not identified. The bactericidal activity against B. subtilis was immediately apparent following the addition of the Natto peptide, and the cells died because of a direct membrane injury (Fig. 6). This means that the production of the Natto peptide constitutes a suicide mechanism of B. subtilis group bacteria. Its mode of action has not been characterized and should be the focus of future research.
Hemolysate or hemin was required for the antimicrobial activity of the Natto peptide against both S. pneumoniae and B. subtilis group strains (Table 4). Heme/hemin could alter the secondary structure of the peptide; however, further examination is necessary to verify this hypothesis.
In conclusion, we identified a unique antimicrobial peptide with a narrow spectrum of activity in Natto extract. S. pneumoniae has great impact in the clinic. It causes acute otitis media and sinusitis mainly in children, community-acquired pneumonia mainly in elders, and meningitis as an invasive infection (Hamborsky et al. 2015). Vaccines against S. pneumoniae are used for the prevention of invasive infection in children and community-acquired pneumonia in elders. Furthermore, antimicrobial resistant S. pneumoniae, such as PRSP, are increasing, and their treatment is now problematic. S. pneumoniae-specific antimicrobials would be promising therapeutic agents, as such agents would not disturb the normal flora; however, peptides are usually difficult to employ as therapeutic agents because of their potentially low stability and bioavailability. Thus the molecular mechanisms of the anti-pneumococcal activity of the Natto peptide should be evaluated for application in therapeutic agents for S. pneumoniae infection.
Authors’ contributions
MK, TSh, SYa, RK, YO, TSa and SYo performed microbiological experiments. HW and HI prepared and analyzed the peptide. MK. TSh, AM and SYo wrote and edited the manuscript. All authors read and approved the final manuscript.
Acknowledgements
The authors thank Dr. Ernesto García for provision of S. pneumoniae R6 and P103 (ΔlytA), and Emiko Suzuki and Hirohisa Okushima for technical assistance in TEM observations.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
We conducted experiments and data were generated. All relevant data are presented in the manuscript.
Funding
This study was supported, in part, by a Grant-in-Aid for Scientific Research (15K08103) from the Japan Society for the Promotion of Science.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CBB
Coomassie Brilliant Blue
- FIC
fractional inhibitory concentration
- MH
Mueller–Hinton
- MIC
minimum inhibitory concentration
- MRSA
methicillin-resistant Staphylococcus aureus
- PRSP
penicillin-resistant Streptococcus pneumoniae
- SDS-PAGE
sodium dodesyl sulfate–polyacrylamide gel electrophoresis
- TEM
transmission electron microscopy
- TH
Todd-Hewitt
- VRE
vancomycin-resistant Enterococcus
Contributor Information
Manabu Kitagawa, Email: kitamana@sapmed.ac.jp.
Tsukasa Shiraishi, Email: shiraishi1020@sapmed.ac.jp.
Soh Yamamoto, Email: sohyama@sapmed.ac.jp.
Ryosuke Kutomi, Email: kutomin@yahoo.co.jp.
Yasuo Ohkoshi, Email: y-okoshi@sapmed.ac.jp.
Toyotaka Sato, Email: sato.t@sapmed.ac.jp.
Hideki Wakui, Email: wakui517@gipc.akita-u.ac.jp.
Hideaki Itoh, Email: itohh@gipc.akita-u.ac.jp.
Atsushi Miyamoto, Email: atsushi@sapmed.ac.jp.
Shin-ichi Yokota, Email: syokota@sapmed.ac.jp.
References
- Bai XH, Chen HJ, Jiang YL, Wen Z, Huang Y, Cheng W, Li Q, Qi L, Zhang JR, Chen Y, Zhou CZ. Structure of pneumococcal peptidoglycan hydrolase LytB reveals insights into the bacterial cell wall remodeling and pathogenesis. J Biol Chem. 2014;289:23403–23416. doi: 10.1074/jbc.M114.579714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari V, Ahmod NZ, Shah HN, Gupta RS. Molecular signatures for Bacillus species: demarcation of the Bacillus subtilis and Bacillus cereus clades in molecular terms and proposal to limit the placement of new species into the genus Bacillus. Int J Syst Evol Microbiol. 2013;63:2712–2726. doi: 10.1099/ijs.0.048488-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Dabbagh F, Negahdaripour M, Berenjian A, Behfar A, Mohammadi F, Zamani M, Irajie C, Ghasemi Y. Nattokinase: production and application. Appl Microbiol Biotechnol. 2014;98:9199–9206. doi: 10.1007/s00253-014-6135-3. [DOI] [PubMed] [Google Scholar]
- De Las Rivas B, García JL, López R, García P. Purification and polar localization of pneumococcal LytB, a putative endo-β-N-acetylglucosaminidase: the chain-dispersing murein hydrolase. J Bacteriol. 2002;184:4988–5000. doi: 10.1128/JB.184.18.4988-5000.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamborsky J, Kroger A, Wolfe S. Pneumococcal disease. In: Hamborsky J, Kroger A, Wolfe S, editors. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. 13. Washington DC: Public Health Foundation; 2015. pp. 279–295. [Google Scholar]
- Hatakeyama S, Kafuku M, Okamoto T, Kakizaki A, Shimasaki N, Fujie N, Takahashi S, Nakayama M, Tagawa H, Komatsuda A, Grave E, Wakui H, Itoh H. Studies on the anticancer mechanisms of the Natto extract. J Soc Mater Eng Resour Jpn. 2016;27:15–19. [Google Scholar]
- Maestro B, Sanz JM. Choline binding proteins from Streptococcus pneumoniae: a dual role as enzybiotics and targets for the design of new antimicrobials. Antibiotics. 2016;5:21. doi: 10.3390/antibiotics5020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestro B, González A, García P, Sanz JM. Inhibition of pneumococcal choline-binding proteins and cell growth by esters of bicyclic amines. FEBS J. 2007;274:364–376. doi: 10.1111/j.1742-4658.2006.05584.x. [DOI] [PubMed] [Google Scholar]
- Mattar EH, Almehdar HA, Yacoub HA, Uversky VN, Redwan EM. Antimicrobial potentials and structural disorder of human and animal defensins. Cytokine Growth Factor Rev. 2016;28:95–111. doi: 10.1016/j.cytogfr.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Moscoso M, Esteban-Torres M, Menéndez M, García E. In vitro bactericidal and bacteriolytic activity of ceragenin CSA-13 against planktonic cultures and biofilms of Streptococcus pneumoniae and other pathogenic streptococci. PLoS ONE. 2014;9:e101037. doi: 10.1371/journal.pone.0101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolosi RJ, Wilson TA, Lawton C, Handelman GJ. Dietary effects on cardiovascular disease risk factors: beyond saturated fatty acids and cholesterol. J Am Coll Nutr. 2001;20:421S–427S. doi: 10.1080/07315724.2001.10719179. [DOI] [PubMed] [Google Scholar]
- O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O’Brien F, Flynn K, Casey PG, Munoz JA, Kearney B, Houston AM, O’Mahony C, Higgins DG, Shanahan F, Palva A, de Vos WM, Fitzgerald GF, Ventura M, O’Toole PW, van Sinderen D. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci USA. 2011;108:11217–11222. doi: 10.1073/pnas.1105380108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano T, Notsumoto S, Nagaoka T, Morimoto A, Fujimoto K, Masuda S, Suzuki Y, Hirauchi K. Measurement of K vitamins in food by high-performance liquid chromatography with fluorometric detection. Vitamins. 1988;62:393–398. doi: 10.1016/0378-4347(89)80012-x. [DOI] [PubMed] [Google Scholar]
- Sang Y, Blecha F. Antimicrobial peptides and bacteriocins: alternatives to traditional antibiotics. Anim Health Res Rev. 2008;9:227–235. doi: 10.1017/S1466252308001497. [DOI] [PubMed] [Google Scholar]
- Schägger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Suarez-Carmona M, Hubert P, Delvenne P, Herfs M. Defensins: “Simple” antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev. 2015;26:361–370. doi: 10.1016/j.cytogfr.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Sumi H, Hamada H, Nakanishi K, Hiratani H. Enhancement of the fibrinolytic activity in plasma by oral administration of nattokinase. Acta Haematol. 1990;84:139–143. doi: 10.1159/000205051. [DOI] [PubMed] [Google Scholar]
- Turenne CT, Snyder JW, Alexander DC. Bacillus and other aerobic endspore-forming bacteria. In: Carroll KC, Funke G, Landry ML, Richter SS, Warnock DW, editors. Manual of clinical microbiology. 11. Washington DC: ASM Press; 2015. pp. 441–461. [Google Scholar]
- Wang G, Mishra B, Epand RF, Epand RM. High-quality 3D structures shine light on antibacterial, anti-biofilm and antiviral activities of human cathelicidin LL-37 and its fragments. Biochim Biophys Acta. 2014;1838:2160–2172. doi: 10.1016/j.bbamem.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DC, Granick S. Hemin biosynthesis in Haemophilus. J Bacteriol. 1963;85:842–850. doi: 10.1128/jb.85.4.842-850.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A. The human cathelicidin LL-37-A pore-forming antibacterial peptide and host-cell modulator. Biochim Biophys Acta. 2016;1858:546–566. doi: 10.1016/j.bbamem.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Yount NY, Bayer AS, Xiong YQ, Yeaman MR. Advances in antimicrobial peptide immunobiology. Biopolymers. 2006;84:435–458. doi: 10.1002/bip.20543. [DOI] [PubMed] [Google Scholar]
- Zaheer K, Humayoun Akhtar M. An updated review of dietary isoflavones: nutrition, processing, bioavailability and impacts on human health. Crit Rev Food Sci Nutr. 2017;57:1280–1293. doi: 10.1080/10408398.2014.989958. [DOI] [PubMed] [Google Scholar]
- Zanetti M, Gennaro R, Skerlavaj B, Tomasinsig L, Circo R. Cathelicidin peptides as candidates for a novel class of antimicrobials. Curr Pharm Des. 2002;8:779–793. doi: 10.2174/1381612023395457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We conducted experiments and data were generated. All relevant data are presented in the manuscript.


