Figure 3.
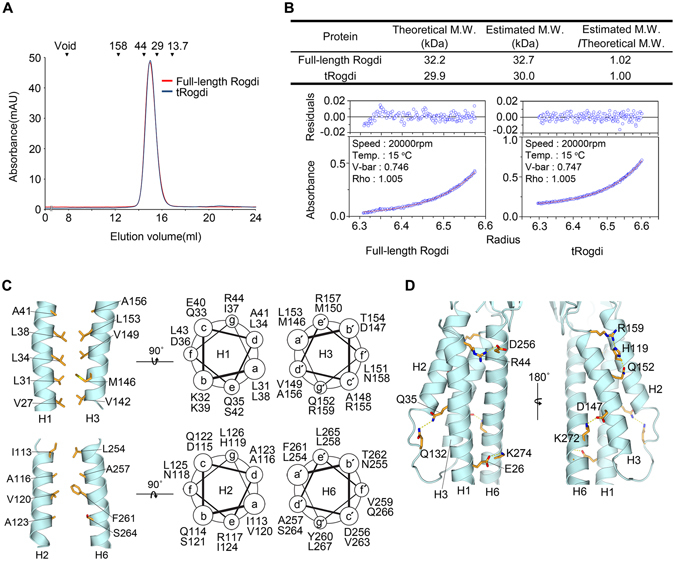
The Leucine Zipper-Like Domain of Rogdi. (A) Size-exclusion chromatography analysis of full-length and truncated (residues 11–276) Rogdi showing the oligomeric state. Molecular mass standards for SEC experiments (top) were aldolase (158 kDa), ovalbumin (44 kDa), carbonic anhydrase (29 kDa) and RNase A (13.7 kDa). Chromatography was performed on a Superdex 200 column in buffer containing 25 mM TRIS, 150 mM NaCl, and 5 mM DTT (pH 7.5). (B) Equilibrium fitting of the results of analytical ultracentrifugation for full-length (left) and truncated (right) Rogdi. The lower panel depicts the fitted overlay (red line) to the experimental data (blue circles). The upper panel depicts the residuals. (C) Helical wheel representation and cartoon diagrams showing the heptad repeat and intermolecular interactions within two parallel helices (H1/H3, top and H2/H6, bottom) of the Rogdi α domain. (D) Detailed view of the intermolecular hydrogen bonds between the surface of amphipathic helices of the α domain. Dotted lines indicate hydrogen bonds (Q35-Q132, R159-H119, and H119-Q152) and three ion pairs (see text for details).
