Figure 1.
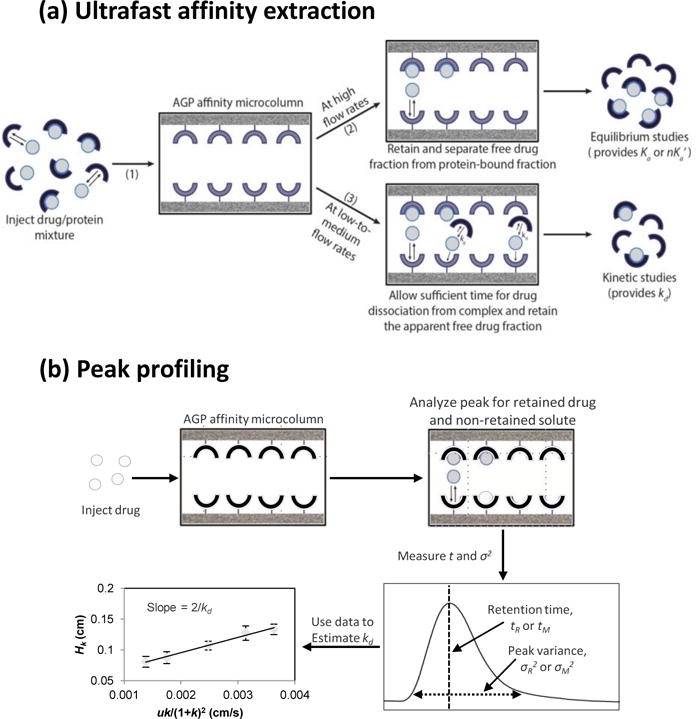
General schemes employed in this study for studying drug-protein interactions by using (a) ultrafast affinity extraction or (b) peak profiling. The method of ultrafast affinity extraction is described in more detail in Sections 3.1–3.3, and the technique of peak profiling is discussed in Section 3.4. Terms: Ka, association equilibrium constant; nKa’, global affinity constant; kd, dissociation rate constant; tR, retention time; tM, void time (i.e., elution time of a non-retained solute); σR2, peak variance of a retained solute; σ2M, peak variance of a non-retained solute; Hk, plate height for a drug or solute due to stationary phase mass transfer; u, linear velocity; k, retention factor.
