Highlights
-
•
Hepatoid carcinoma of the ovary is rapidly progressing rare epithelial ovarian tumor.
-
•
The term ‘hepatoid’ refers to the morphological resemblance with hepatocellular carcinoma.
-
•
Final diagnosis is made via positive staining for alpha fetoprotein along with immunohistochemistry
-
•
Combination chemotherapy can be considered superior over the targeted agents while treating this tumor.
1. Introduction
Hepatoid carcinoma of the ovary is an uncommon and aggressive neoplasm which typically is reported in elderly women in the advanced stages (Randolph et al., 2015). WHO defines Hepatoid ovarian carcinoma (HCO) as an adenocarcinoma with morphologic characteristics similar to hepatocellular carcinoma, arising from an anatomic site other than the liver. These tumors can arise from various body sub-sites most commonly in stomach and less commonly in ovaries and uterus, lungs, bladder and kidneys (Lazaro et al., 2007). The tumor is mainly composed of epitheloid cells with elevated AFP levels (Wang et al., 2013). This tumor has distinguishable features when compared with other primary ovarian tumors as well as hepatocellular carcinoma (Pandey & Truica, 2011). These tumors are highly aggressive and have poor prognostic outcome with median overall survival of 2 years (Lazaro et al., 2007). There is no definitive treatment strategy for this tumor type and literature review showed various therapies including cytoreductive surgery, chemotherapy and targeted therapy still under investigation. Our patient is a 41 year old female who presented with left adnexal mass and omental and peritoneal nodularity with elevated serum AFP and CA-125 levels. She was diagnosed as metastatic hepatoid carcinoma of the ovary. Such a case has not been previously reported in Pakistan.
2. Case report
A 41 year old female patient diabetic and hypertensive, with one alive child and no family history of any malignancy presented with the complaints of relative constipation for the past one year associated with mild lower abdominal pain and distension, anorexia and secondary infertility. She had a past history of left ovarian cystectomy 6 years ago. On histopathology, the cyst grossly measured 6 × 5 cm and on cut surface it contained gelatinous material with smooth inner surface. Microscopically it showed low cuboidal cystic lining representing Serous Cystadenoma.
Patient developed afore mentioned symptoms along with secondary infertility for which she was investigated. CT scan abdomen and pelvis showed extensive metastatic disease in abdomen and pelvis in the form of multiple omental and peritoneal deposits (Fig. A), one of the lesions seen in left adnexa measuring approximately 3 × 5 cm in longitudinal and transverse dimensions respectively with nonvisualization of left ovary (Fig. B). Hepatomegaly with tiny hypodense lesion in left lobe was seen with trace of perihepatic and perisplenic fluid. Mild left pleural effusion, ground glass haze and multiple nodules were seen in bilateral basal lungs. Hysterosalpingography for secondary infertility was also done that concluded smooth indentation on the fundal uterine cavity suggestive of arcuate uterus. There was slight irregular endometrial cavity with small contrast opacified outpouchings, predominantly in fundal region, suggestive of adenomyosis. Fallopian tubes were patent.
Fig. A.

Pretreatment CT scan showing extensive omental and peritoneal deposits.
Fig. B.

Pretreatment CT scan showing left sided Adnexal mass of approximately 3 × 5 cm in size.
Core biopsy of omental nodule was done that revealed cores of fibrocollagenous tissue involved by the tumor with delicate vascularized connective tissue network. Tumor was composed of round to polygonal cells with prominent cell membrane, eosinophilic granular to vacuolated cytoplasm and centrally located hyperchromatic nuclei with pleomorphism. Mitotic activity was noted. Immunohistochemical stains Hepatocyte Paraffin 1, Arginase, Glypican, and CKAE1/AE3 were positive in tumor cells while SALL 4, PAX 8 and Inhibin were negative. Morphology and immunohistochemical profile were consistent with the diagnosis of metastatic hepatocellular carcinoma.
Biopsy report was correlated with Triphasic CT scan of the liver which showed smooth hepatic contours without any significant heterogeneity of hepatic parenchyma (Fig. C). No left lobar or caudate hypertrophy was seen to suggest cirrhosis. No focal intrahepatic arterially enhancing lesion was seen to suggest HCC. No washout was seen on portovenous and delayed phases. Extensive omental disease with hepatic serosal deposits with further soft tissue masses along the serosal surface of gastric antrum which too represented a peritoneal disease was reported.
Fig. C.

Sections from Triphasic CT scan liver showing normal liver parenchyma.
Patient was then referred to Oncology department of Nuclear Medicine Oncology and Radiotherapy Institute (NORI) where she was evaluated. On examination patient had good performance status (ECOG 1). Abdomen was distended and tense, no visceromegaly or lymphadenopathy was palpable. Tumor markers were done which were raised CA-125 (114.7 U/ml) and AFP levels (335.9 IU/ml) while CEA levels were within normal limits (3.90 ng/ml). Histopathology slide review was done and in light of clinical details provided i.e. normal CT scan of the liver and raised AFP and CA-125 levels the diagnosis of Metastatic hepatoid carcinoma of the ovary was made.
The case was discussed in multidisciplinary meeting with gynecologists and optimal cytoreductive surgery was not possible due to extensive omental and peritoneal deposits. As the disease resembled clinically and morphologically to hepatocellular carcinoma, the patient was planned to be treated on the same lines as HCC. Patient was started on Oral tyrosine kinase inhibitor Sorafenib after all baseline investigations but the disease progressed further after 2 months of treatment with Sorafenib (Fig. D, Fig. E) and the patient was shifted to treatment with combination chemotherapy Paclitaxel and Carboplatin. After 2 cycles of chemotherapy patient was subjectively improved. Tumors markers AFP and CA-125 dropped down to 178 IU/ml and 39 IU/ml respectively. Interim radiological assessment also showed improvement in the disease process (Fig. F, Fig. G). According to RECIST criteria partial response was observed.
Fig. D.

CT Scan pelvis after 2 months of treatment with Sorafenib showing disease progression
Fig. E.
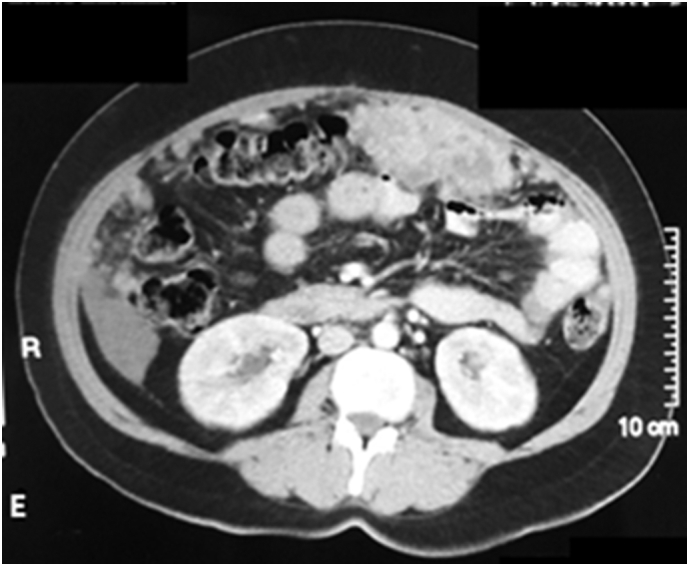
CT Scan abdomen after 2 months of treatment with Sorafenib showing disease progression.
Fig. F.
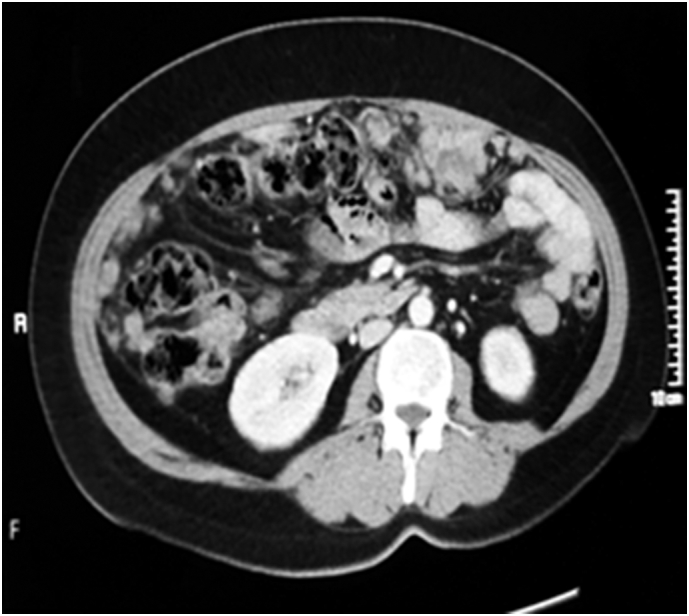
CT scan abdomen after 2 cycles of treatment with Paclitaxel/Carboplatin showing regression of the disease process.
Fig. G.

CT scan pelvis after 2 cycles of treatment with Paclitaxel/Carboplatin showing regression of the disease process.
3. Discussion
Hepatoid carcinoma is a rare malignant epithelial tumor with aggressive clinical behavior. It is usually diagnosed in the age range of 42 to 78 years (average age of 62 years) not only affecting post-menopausal women but also middle-age female population (Pandey & Truica, 2011). In our case the lady was 41 years old and was diagnosed with the disease during her investigation process for secondary infertility.
In an article published in 1987, Ishikura and Scully described Hepatoid carcinoma of the ovary for the first time in which the tumor cells resembled Hepatocellular carcinoma morphologically and immunohistochemically. Five cases were reported in which tumors presented as adnexal masses containing moderate to abundant eosinophilic cytoplasm and cellular arrangement in sheets and stained positive for alpha-fetoprotein (Ishikura & Scully, 1987). In our case tumor cells showed same morphology.
HCO must be differentiated from Hepatic yolk sac tumor (HYST) and Hepatocellular carcinoma (HCC). HYST usually affects younger population and is regarded as a germ cell tumor and stains negative for Hepatocyte paraffin 1 and focally positive for CEA. The presence of bile, microscopic findings and negativity for CA-125 helps distinguish HCC from HCO (Pandey & Truica, 2011). Hepatocyte Paraffin 1 helps distinguish hepatocytic from non-hepatocytic tumors but can't differentiate it from HYST and HCC (Pitman et al., 2004). SALL4 is considered to be a diagnostic marker in germ cell tumors and stains negative in HCC but shows positivity in hepatoid carcinomas of gastric origin (Ushiku et al., 2010) but was negative in our case.
Immunohistochemistry of the tumor in our patient showed positivity for Hepatocyte Paraffin 1, Glypicans, and Keratins CKAE1/AE3 while tissue sample was negative for SALL4, PAX8 and Inhibin.
These tumors can commonly be bilateral (22% cases) at presentation and mostly diagnosed in advanced stages III and IV (Pandey & Truica, 2011). Our patient at the time of presentation had left sided adnexal mass with extensive peritoneal, omental and hepatic serosal deposits making it an advanced disease. In terms of prognostic outcome, rapid progression is characteristic with overall survival of one and two years in the range of 83% and 53% respectively (Randolph et al., 2015).
The absence of any hepatic lesion on radiological imaging and marked elevation of AFP confirms ectopic hepatoid origin of the tumor. Raised CA-125 levels specify the ovarian epithelial origin. Although AFP levels are much more markedly raised as compared to CA-125 (Isonishi et al., 2009) which makes it non-specific marker in case of hepatoid carcinomas (Pandey & Truica, 2011). CEA can't be considered as a useful marker as it can be negative or focally positive. In our case same pattern was observed.
Hepatoid Adenocarcinomas have poor prognostic outcome with median survival of 12 months and most of the tumors have already metastasized at the time of diagnosis (Tong et al., 2016). There is no definitive standard treatment protocol for this rare cancer. There has been a controversy whether patient should be treated on the lines of ovarian epithelial tumor or as hepatocellular carcinoma due to its pathological resemblance with it. In one study cytoreductive surgery was done followed by adjuvant treatment (Campos et al., 2013) while another patient was treated as a primary ovarian cancer with dose-dense paclitaxel and carboplatin (Randolph et al., 2015). In another study Tyrosine kinase inhibitor Sorafenib was given to the patient as second line treatment but was discontinued as AFP levels increased and disease progressed (Pandey & Truica, 2011).But in another case reported Sorafenib was able to achieve 7 months of progression free survival (Petrelli et al., 2012). Our patient had been started treatment with Sorafenib, which is also approved in treatment of advanced hepatocellular carcinomas. Patient had been under treatment for two months but progression of the disease occurred and patient was shifted to combination of paclitaxel and carboplatin. She has responded well to combination chemotherapy with remarkable subjective, radiological and biochemical response.
4. Conclusion and recommendation
The hepatoid carcinomas of the ovary can present in middle aged females. The final diagnosis is made through radiological imaging and histopathology along with immunohistochemical profile of the tumor. Pathologists should report carefully keeping rare disease presentations in mind so that the diagnosis is not missed and appropriate treatment can be given. Paclitaxel and carboplatin combination can be tried as first line chemotherapy.
Conflicts of interest
There are no conflicts of interest.
References
- Randolph L.K., Hopkins M.K., Hopkins M.P., Wasdehl D.A. Hepatoid carcinoma of the ovary: a case report and review of the literature. Gynecol. Oncol. Rep. Aug 2015;13:64–67. doi: 10.1016/j.gore.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro J., Rubio D., Repolles M., Capote L. Hepatoid carcinoma of the ovary and management. Acta Obstet. Gynecol. Scand. 2007;86(4):498–499. doi: 10.1080/00016340600593117. [DOI] [PubMed] [Google Scholar]
- Wang L., Zhong Y., Sun L., Zhou H., Chen W., Zhang X. Clinical and pathological features of hepatoid carcinoma of the ovary. World J. Surg. Oncol. 2013 Jan 30;11:29. doi: 10.1186/1477-7819-11-29. doi: 10/1186/1477-7819-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey M., Truica C. Hepatoid carcinoma of the ovary. J. Clin. Oncol. May 20, 2011;29(15):e446–e448. doi: 10.1200/JCO.2010.33.6321. [DOI] [PubMed] [Google Scholar]
- Ishikura H., Scully R.E. Hepatoid carcinoma of the ovary: a newly described tumor. Cancer. 1987;60:2775–5784. doi: 10.1002/1097-0142(19871201)60:11<2775::aid-cncr2820601130>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Isonishi S., Ogura A., Kiyokawa T., Suzuki M., Kunito S., etal Hirama M. Alpha-fetoprotein (AFP)-producing ovarian tumor in an elderly woman. Int. J. Clin. Oncol. 2009 Feb;14(1):70–73. doi: 10.1007/s10147-008-0800-4. [DOI] [PubMed] [Google Scholar]
- Pitman M.B., Triratanachat S., Young R.H., Oliva E. Hepatocyte paraffin 1 antibody does not distinguish primary ovarian tumors with hepatoid differentiation from metastatic hepatocellular carcinoma. Int. J. Gynecol. Pathol. Jan 2004;23(1):58–64. doi: 10.1097/01.pgp.0000101141.31270.a1. [DOI] [PubMed] [Google Scholar]
- Ushiku T., Shinozaki A., Shibahara J., Iwasaki Y., Tateishi Y., Funata N. SALL4 represents fetal gut differentiation of gastric cancer, and is diagnostically useful in distinguishing hepatoid gastric carcinoma from hepatocellular carcinoma. Am. J. Surg. Pathol. Apr 2010;34(4):533–540. doi: 10.1097/PAS.0b013e3181d1dcdd. [DOI] [PubMed] [Google Scholar]
- Tong Ling, Pan Huaxiong, He Jun, Weng Mixia, Zheng Liduan. Hepatoid adenocarcinoma arising from heterotopic pancreas of the ileum: a case report. Medicine. August 2016;95(33):e4067. doi: 10.1097/MD.0000000000004067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos P., Martinez J., Torroba A., Machado F., Paricio P. Peritoneal dissemination of hepatoid carcinoma of the ovary treated with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy. Medicine. 2013;2013:283295. doi: 10.1155/2013/283295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrelli F., Ghilardi M., Colombo S., Stringhi E., Barbara C., etal Cabiddu M. A rare case of metastatic pancreatic hepatoid carcinoma treated with sorefenib. J. Gastrointest. Cancer. Mar 2012;43(1):97–102. doi: 10.1007/s12029-011-9264-2. [DOI] [PubMed] [Google Scholar]


