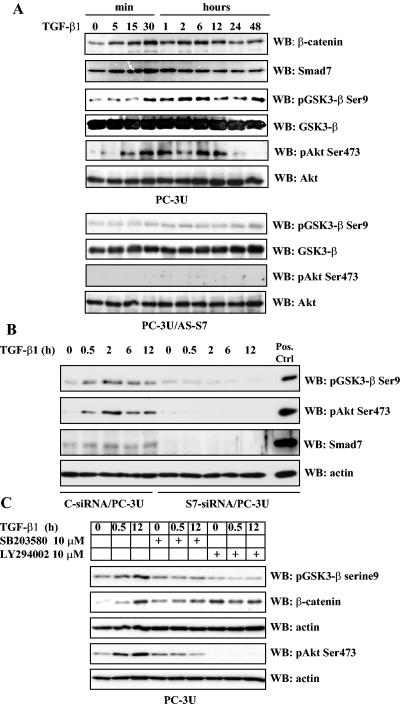
FIG. 5.
TGF-β regulates the levels of β-catenin in PC-3U cells via phosphorylation of GSK-3β on Ser9 and Akt on Ser473 in a Smad7-, p38 MAP kinase-, and phosphatidylinositol 3-kinase-dependent manner. Total cell lysates were prepared from PC-3U and PC-3U/AS-S7 cells, treated or not with TGF-β, and used for immunoblotting. (A) Levels of β-catenin and Smad7 and of GSK-3β and Akt phosphorylated at Ser9 and Ser473, respectively, in PC-3U cells treated with TGF-β for different time periods. In the upper and lower panels, the phosphorylated and nonphosphorylated forms, respectively, of GSK-3β and Akt, are shown. A similar analysis with PC-3U/AS-S7 cells treated with TGF-β is also shown. Equal amounts of proteins were loaded for each cell line. The filters were subjected to immunoblotting simultaneously under equal conditions. (B) Lysates from PC-3U cells transiently transfected with control (C-siRNA) or specific Smad7 siRNAs (S7-siRNA) were treated or not with TGF-β for the indicated time periods, and the total cell lysates were investigated for the amount of endogenous pGSK-3β Ser9, phospho-Akt Ser473, and Smad7. The filters were then stripped and reprobed with actin antibodies to confirm equal loading of proteins. Total cell lysate from cells ectopically expressing Flag-Smad7 (PC-3U/pMEP4S-7) were used as the positive control for Smad7 (Pos. Ctrl). (C) Effects of the p38 inhibitor SB203580 and the phosphatidylinositol 3-kinase inhibitor LY294002 on TGF-β-induced phosphorylation of GSK-3β on Ser9. Total cell lysates were prepared from PC-3U cells treated or not with TGF-β, in the presence or absence of SB203580 and LY294002, andsubjected to immunoblotting. The phosphorylation of GSK-3β on Ser9 and amount of β-catenin are shown in the upper two panels, and the phosphorylation of Akt on Ser473 is shown in the lower panel. Both filters were stripped, blocked, and reprobed with actin antibodies, which served as the control for equal loading of proteins.