Figure 3.
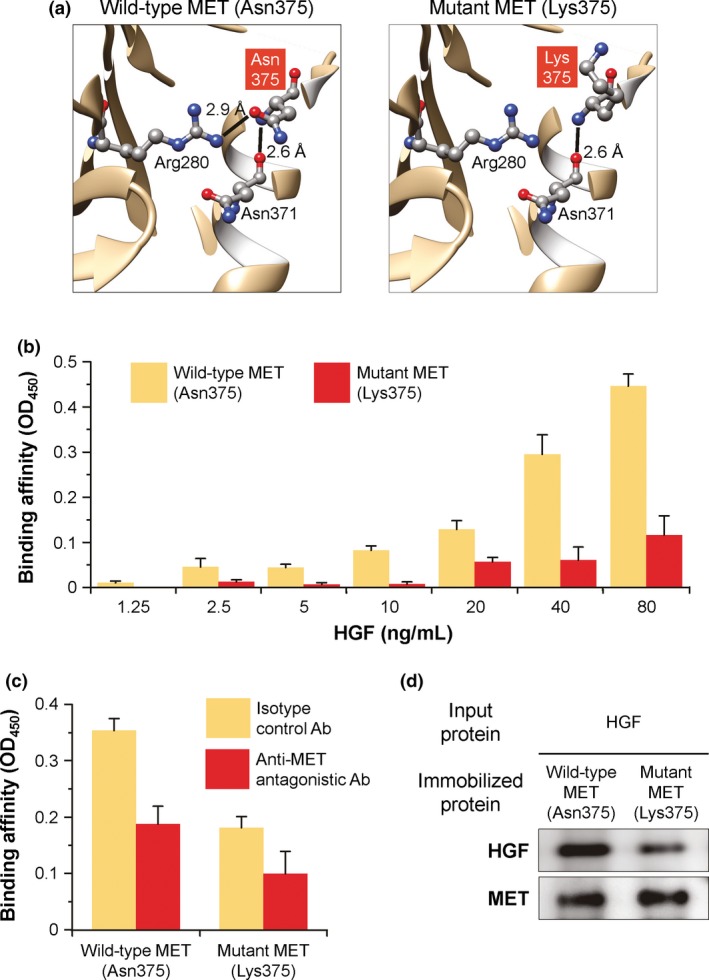
Structure and function of wild‐type (Asn375) and mutant (Lys375) MET ectodomains. (a) Ribbon representation of the MET ectodomains. Asn375 and Lys375 are shown with the surrounding residues by ball‐and‐stick models. Lines represent hydrogen bonds with the distances; gray, carbon; red, oxygen; blue, nitrogen. (b) HGF‐binding capacity of the MET ectodomains. MET‐Fc fusion protein was immobilized to ELISA plates and incubated with increasing concentrations of recombinant human HGF protein. The bound HGF was detected by anti‐HGF Ab and colorimetrically quantified as MET‐HGF binding affinity by the absorbance value at OD 450. (c) Binding specificity of the MET ectodomains to HGF. The study was identical to that described in (b), except that the immobilized MET‐Fc protein was pretreated with anti‐MET antagonistic Ab or isotype control Ab before incubation with 40 ng/mL HGF. (d) Coprecipitation analysis of the MET ectodomains with HGF. Recombinant human HGF protein was coprecipitated with MET‐Fc fusion protein immobilized on protein G‐Sepharose beads. The precipitates were resolved by SDS‐PAGE and then visualized by immunoblotting with anti‐HGF or anti‐MET Ab. For (b) and (c), data are presented as the mean ± standard error of n = 4 (b) or n = 3 (c) per group.
