FIG. 7.
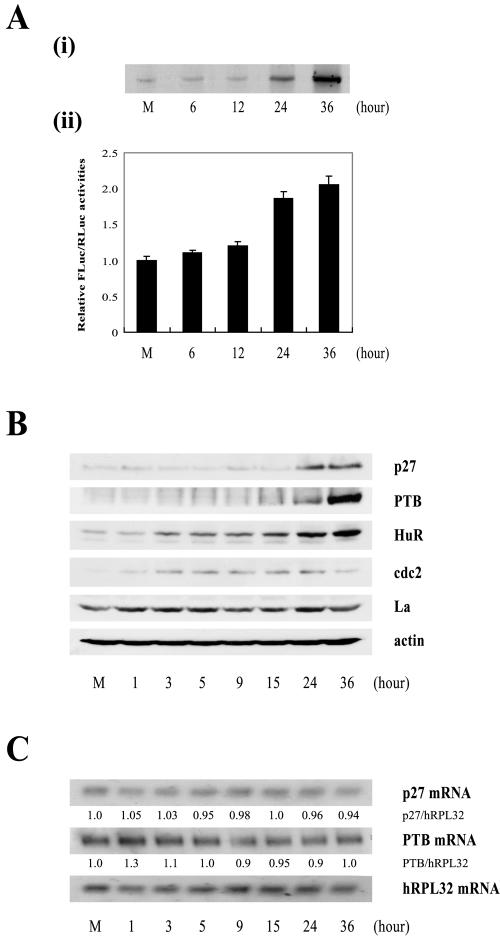
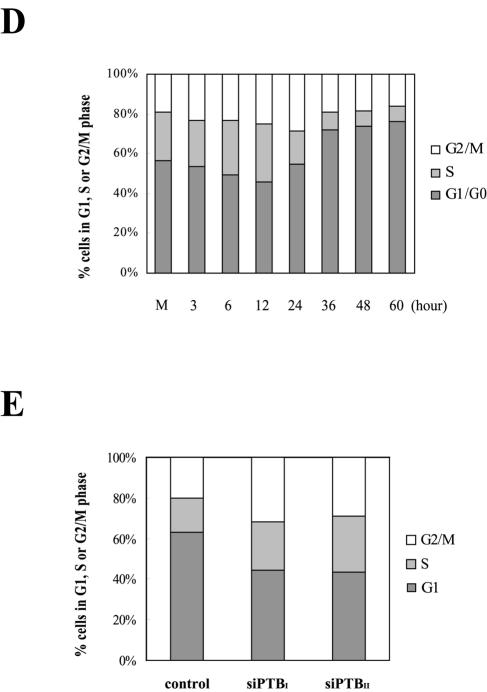
IRES activity of the p27 mRNA during differentiation of HL60 cells. (A, i) The newly synthesized p27 proteins were monitored by metabolic labeling of proteins with [35S]Met and [35S]Cys followed by immunoprecipitation with an antibody against p27. The immunoprecipitated samples were resolved by SDS-15% PAGE and then subjected to autoradiogram. (ii) Differentiation of HL60 cells was induced by TPA treatment (30 nM). The Rp27F dicistronic reporter RNA was transfected into HL60 cells 6, 12, 24, and 36 h after TPA was used to induce differentiation, and luciferase activities were measured 3 h posttransfection. The relative luciferase activity of untreated cells was set to 1. (B) Protein levels in HL60 cells during differentiation. HL60 cells were harvested 1, 3, 5, 9, 15, 24, and 36 h after TPA treatment (30 nM) and then subjected to Western blot analysis with anti-p27, -PTB, -HuR, -cdc2, -La, and -actin antibodies. (C) Northern blot analysis of p27, PTB, and hRPL32 mRNAs during differentiation of HL60 cells. Cells were treated with TPA (30 nM) for 1, 3, 5, 9, 15, 24, and 36 h, and total RNAs were isolated. The RNAs (30 μg each) were resolved on a denaturing agarose gel, transferred to a nylon membrane, and hybridized with 32P-labeled probes for p27, PTB, and hRPL32. Relative radioactivities of bands corresponding to p27 and PTB mRNAs were calculated as described in the legend to Fig. 6D. The relative radioactivities of undifferentiated cells were set to 1. (D) Changes in cell phase population proportions during differentiation of HL60 cells. HL60 cells were stained with propidium iodide before (lane M) and after treatment with TPA (30 nM) for the indicated times (6, 12, 24, 36, 48, and 60 h) and then subjected to FACS analysis. The relative proportions of cells in the different phases (G2/M, S, and G1/G0) are depicted in the graphs. (E) Effects of PTB-specific siRNAs on cell proliferation. The proportion of cell phases in control and siRNA-expressing cells was analyzed and depicted as described for panel D.