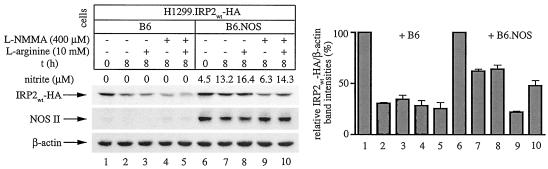
FIG. 7.
Stabilization of IRP2 by physiologically generated NO via intercellular signaling. H1299 cells (106) expressing IRP2wt-HA (indicated as H1299.IRP2wt-HA) were mixed with either 3 × 106 control B6 cells (lanes 1 to 5) or 3 × 106 B6.NOS cells (lanes 6 to 10) and evenly plated onto 100-mm-diameter dishes. The cells were cultivated in tetracycline (tet)-free media to activate the expression of IRP2wt-HA. After 24 h, 5 mM sodium butyrate was added and incubation was continued for another 12 h to augment the expression of NOS II (22). Subsequently, tetracycline (2 μg/ml) was added back to turn off the synthesis of IRP2wt-HA, and, after an 1 h incubation, the cells were either harvested (lanes 1 and 6) or further incubated for 8 h under following conditions: no additives (lanes 2 and 7), with 10 mM l-arginine (lanes 3 and 8), or with 400 μM l-NMMA in the absence (lanes 4 and 9) or presence of 10 mM l-arginine (lanes 5 and 10) to modulate NO production. Nitrite levels in the culture supernatant were measured with Griess reagent. The levels of IRP2wt-HA (expressed in H1299.IRP2wt-HA cells), NOS II (expressed in B6.NOS cells), and control β-actin (expressed in H1299.IRP2wt-HA, B6.NOS, and control B6 cells) were analyzed by Western blotting with HA (top), NOS II (middle), and β-actin antibodies (bottom). The immunoreactive bands were quantified by densitometric scanning. The IRP2wt-HA/β-actin ratios from three independent experiments (± standard deviations) are plotted at the bottom. t, time.